Meeting Summary
Renal denervation (RDN) is a minimally invasive procedure used as an adjunctive treatment for individuals with resistant hypertension when blood pressure (BP) remains uncontrolled despite medications and lifestyle changes. Currently, medical inertia and poor patient adherence are the two major reasons for poor control of hypertension, and BP control is particularly challenging in patients with resistant hypertension. There are limitations to the current guideline recommendations of combination therapy as first-line treatment, and available strategies that rely on lifestyle changes and antihypertensive therapy do not achieve a reduction in BP below 140/90 mmHg in the global trial population. Here, the studies within published meta-analyses are investigated when assessing the viability of RDN as an adjunctive therapy for uncontrolled hypertension to make informed decisions for how to treat specific patient groups.
The Great Debate: Renal Denervation is One of the Pillars of Management of Resistant Hypertension
Introduction
The past decade has seen some considerable modifications in the approach to the treatment of hypertension, a condition that remains an important risk factor for major cardiovascular events and mortality.1 Despite therapeutic advances and updated guidelines, conventional strategies involving lifestyle changes and pharmacotherapy often fail to control BP below target levels in a significant portion of patients.2 Recent clinical interest has focused on RDN, a device-based procedure, as a potential adjunctive therapy for patients with uncontrolled or resistant hypertension.3
This article summarises the Great Debate session recorded at the European Society of Cardiology (ESC) Congress, held from 29th August–1st September 2025 in Madrid, Spain, which addressed the position of RDN for uncontrolled and resistant hypertension in patients with estimated glomerular filtration rate (eGFR) ≥40 who should be referred to specialist centres.
Evidence Supporting Renal Denervation
Dagmara Hering, Medical University of Gdansk, Poland, began the session with insights from the observational longitudinal EnligHTN study, which revealed that hypertension is inadequately controlled in around 70% of patients (USA: n=23, 439/34,009 [68.9%]; UK: n=204, 104/274,986 [74.2%]).3,4 Poor control is largely due to medical inertia and low patient adherence to medication.5 Hering presented previous investigations of drug monitoring in different cohorts of patients with apparently resistant hypertension to support this, showing that, on average, 17% of patients were not adherent to therapy and 44% were partially adherent to the prescribed medication.5
While single-pill combination therapy has improved rates of BP control to <140/90 mmHg in approximately 66.5% of patients, this does not meet the optimal target (<130/80 mmHg) and leaves patients at risk for BP variability, particularly in the morning, associated with higher cardiovascular risk.2 The efficacy of antihypertensive drugs further diminishes over time, with evidence from a meta-analysis suggesting a 5.1 mmHg reduction in systolic BP persisting for up to 4 years on average.6 Analysis of the long-term SPRINT trial also revealed a beneficial effect on cardiovascular morbidity and all-cause death for 3–4 years. However, at a median follow-up of 8 years, there was no evidence of any beneficial effect of intensive treatment for cardiovascular mortality and all-cause mortality, and no comparable difference between the trial arms regarding systolic BP levels (Figure 1).7
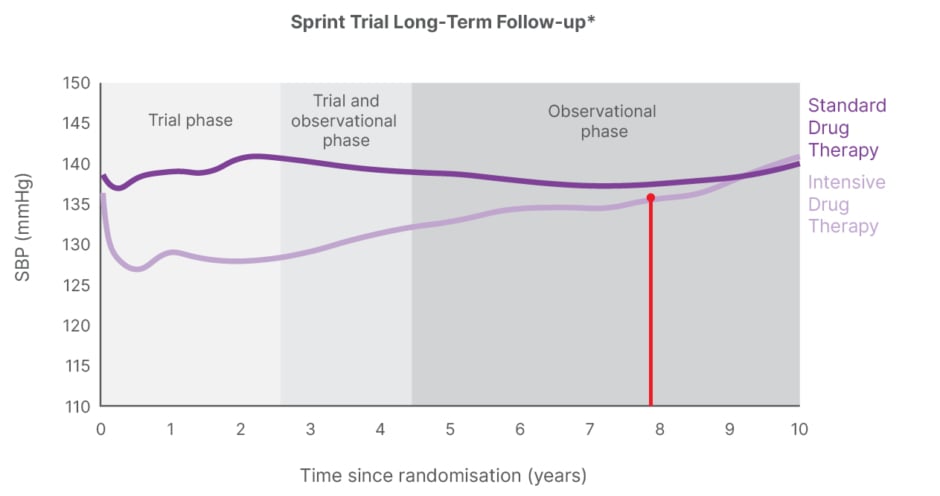
Figure 1: Benefit for cardiovascular mortality or all-cause mortality no longer shown after 8 years.
*Electronic health record data.
Adapted from Jaeger BC et al.7
SBP: systolic blood pressure.
RDN targets the sympathetic nervous system, reducing renin secretion while increasing sodium excretion and renal blood flow. Second-generation, sham-controlled, randomised trials consistently show the procedure to be effective and safe, with robust 24-hour BP reductions among patients who continue (or are withdrawn from) antihypertensive medication, as well as those with resistant hypertension.8-12
Significant BP reductions are observed across subgroups, including those with diabetes, chronic kidney disease, atrial fibrillation (AF), the elderly, and patients with high-risk post-myocardial infarction or stroke.13 International guidelines now recommend RDN for uncontrolled and resistant hypertension, and notably, patients with eGFR ≥40 mL/min/1.73 m² should be referred to specialist centres.14-17
Long-term data from clinical trials and registries from >3,200 patients show sustained BP reductions for up to 3 years18-22 and 5 years after the primary efficacy endpoint was met.23 In a meta-analysis of 18 studies (n=2,212; mean: 4.4 years follow-up), office BP reductions averaged −23 mmHg (15 reports; p<0.05) and systolic ambulatory BP reductions averaged −13.6 mmHg (11 reports; p<0.05). There was also a decrease in the number of prescribed antihypertensive medications and eGFR.24 Unpublished long-term data spanning 8–10 years (n=384) report long-term sustained 24-hour BP reduction after RDN with significant reduction (–15.7 mmHg) in systolic ambulatory BP monitoring and diastolic ambulatory BP monitoring (–9.21 mmHg) at 8.7 years follow-up (Tsioufis et al., unpublished data).
Hering highlighted that the key advantage of RDN is its ‘always on’ effect. It consistently lowers BP, independent of patient adherence, drug kinetics, or dosing.14 Safety data are robust: three meta-analyses report no evidence of compromised renal function, and the incidence of renal stenosis was 0.2%, with no renal artery damage directly related to the procedure.25-27
Evidence Against Routine Use
Franz Messerli, Swiss Cardiovascular Center, University Hospital Bern, Switzerland, began his presentation with a reminder of how some meta-analyses, particularly those sponsored by device manufacturers, show BP reductions of –3.6 mmHg for 24-hour systolic BP, results which perhaps do not under-represent the achievable antihypertensive benefits of RDN.28 Messerli and colleagues also performed a meta-analysis, which demonstrated benefits with both radiofrequency and ultrasound RDN compared with sham trials.29 The 2025 American Heart Association/American College of Cardiology (AHA/ACC) guideline acknowledges small (3–5 mmHg) but significant reductions in 24-hour ambulatory BP in some trials, but not all.17
Stressing the importance of the statement that ‘individual patient response varies widely’,30 Messerli presented data from the SPYRAL HTN-OFF MED proof-of-concept trial, which demonstrated a fan response with up to one-third of patients showing no benefit, or even an increase in arterial pressure after RDN.8 Messerli acknowledged that patients with refractory hypertension, where BP remains uncontrolled despite maximal therapy, are identified as the group most likely to benefit from the procedure.31,32
Although FDA approval for RDN was granted in 2023, Messerli reminded the audience how caution was noted by regulatory leaders, reflecting uncertainty over population-level benefits.31 Historical analogies with surgical sympathectomy also illustrate the unpredictable and often transient efficacy of ablative procedures,33 and he underlined the message that enthusiasm should be tempered by the realisation that the field is still evolving and ongoing investigation is warranted.34
Multidisciplinary and Practical Considerations: Panel and Audience Discussion
Sofie Brouwers, OLV Ziekenhuis, Aalst, Belgium, acknowledged that in debates such as this, the most accurate understanding of a situation is often found by balancing or synthesising two opposing viewpoints, rather than by choosing one extreme over the other.
It was recognised that, while registry and RCT data support efficacy and safety, long-term outcome evidence for cardiovascular events is still lacking, and much existing data are from registries susceptible to selection bias. The existing data may need to be sorted to help distinguish scientific debates (sham-controlled RCTs) from debates based on real-world situations (outcome data from registries). Longer- term data that include placebo-controlled effects are also needed, which could determine any variability in sympathetic neural components and artery remodelling related to the procedure.
Device-related complications are rare (<1%), but not negligible, and strict patient selection remains critical,25-27 with an emphasis made on the need for multidisciplinary selection and ongoing patient engagement. Regarding nonadherent patients, the panel agreed that RDN was a likely option to reduce BP and the risk of future cardiovascular events. Clinicians need to discuss reasons for nonadherence with patients, because if an individual is nonadherent to medication, this might also reveal possible noncompliance to follow-up after the RDN procedure.
The most recent guidelines of the AHA/ACC clearly state that BP reduction is –3 to –5 mmHg and that some patients do not respond.17 Importantly, RDN is not replacing drugs because patients have uncontrolled hypertension. RDN is added to achieve the target BP, which is variable, and highlights the importance of continual observation of patients to monitor symptoms and stabilise the patient accordingly.
Key Takeaways and Concluding Remarks
- RDN is one of the few interventions with a sham-controlled arm in interventional cardiovascular medicine.
- RDN sham-controlled trials and international registries provide evidence for long-term, meaningful BP-lowering efficacy and safety in diverse phenotypes of patients who are hypertensive.
- Current guidelines set the stage.
- RDN fulfils an unmet need in hypertension care, and patients want additional treatment options.
RDN fulfils an unmet need for specific individuals who are hypertensive, particularly those with resistant or refractory disease or a high risk of cardiovascular complications, when implemented with robust multidisciplinary oversight. Hypertension pathophysiology is multifactorial, and there is a need for more long-acting drugs or additional options. Patient screening for trials is complex, and multidisciplinary team collaboration is needed to prevent and reduce cardiovascular events and mortality in participants, and to ensure that selected patients benefit over the longer term. Additionally, long-term cognitive and cardiovascular risk reduction may demand earlier, more comprehensive BP control starting as early as 30–40 years of age.
Therapeutic Effects of Renal Denervation Beyond Arterial Hypertension: A Systematic Review and Meta-Analysis
RDN can be considered as an adjunctive treatment for patients with resistant and uncontrolled hypertension. However, excess sympathetic nervous system activity constitutes an underlying mechanism for many other disorders, as well as arterial hypertension.
This poster session reported on a systematic review and meta-analysis that investigated the therapeutic effects of RDN beyond hypertension. It was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and electronic databases such as PubMed, Embase, and Cochrane Library were searched for eligible studies that assessed RDN effects and AF recurrence, obstructive sleep apnoea (OSA), metabolic parameters, heart failure, and diastolic heart function.
The inclusion criteria incorporated RCTs and observational studies with relevant outcome measures, and effect sizes were pooled using the random effect model. A total of 16 studies comprising 818 patients were included.
The study concluded that RDN was associated with:
- A significant increase in freedom from AF when combined with pulmonary vein isolation (relative risk: 1.30; 95% CI: 1.04–1.61; p=0.35; Figure 2).
- An observed trend in OSA improvement (Figure 2).
- Decreased fasting blood glucose.
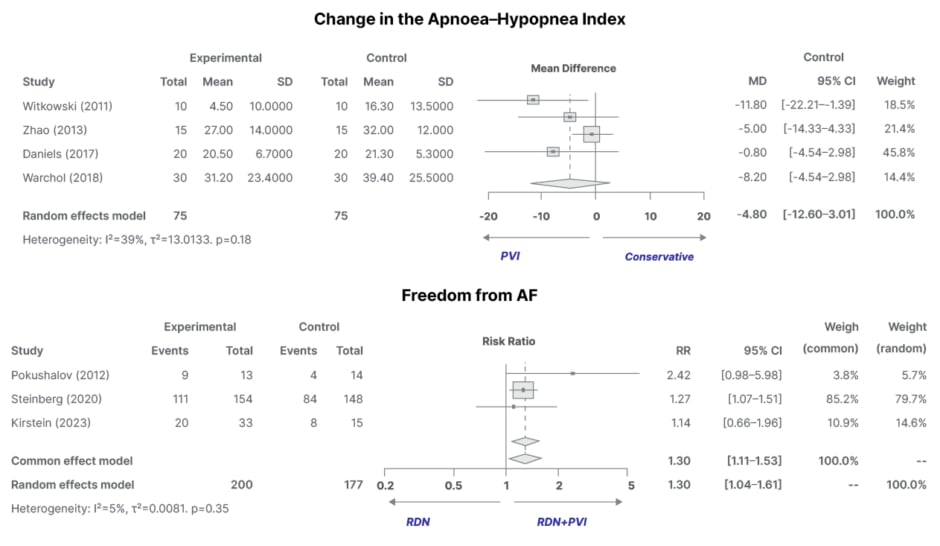
Figure 2: Observed change in obstructive sleep apnoea severity (as measured by the Apnoea–Hypopnea Index) and significant increase in freedom from atrial fibrillation.
AF: atrial fibrillation; PVI: pulmonary vein isolation; RDN: renal denervation; RR: risk ratio.
Additionally, RDN led to improvements in left ventricular diastolic function and improved heart failure-related biomarkers, specifically N-terminal pro-B-type natriuretic peptide and the 6-minute walk test.
Concluding Remarks
This meta-analysis suggests that RDN exhibits beneficial effects beyond hypertension, particularly in AF burden, OSA severity, metabolic parameters, and cardiac function parameters. These findings support the broader role of RDN in BP regulation and cardiovascular health.