BACKGROUND
Hypertrophic cardiomyopathy (HCM) is a progressive primary myocardial disorder characterised by unexplained left ventricular (LV) hypertrophy, often caused by pathogenic variation in genes encoding sarcomeric proteins.1 HCM impacts heart function with characteristically hyperdynamic contraction and impaired compliance as a result of excess myosin–actin cross-bridging. It is estimated that approximately 70% of HCM patients have obstructive HCM, characterised by dynamic LV outflow tract obstruction at rest or with provocation, whereas approximately 30% have non-obstructive HCM (nHCM), with no significant LV outflow tract obstruction (<30 mmHg).2,3 Patients with nHCM can experience debilitating symptoms, but there are no proven pharmacologic therapies available and current options are very limited.3,4
By altering the contractile mechanics of the cardiomyocyte, myosin inhibitors have the potential to modify the pathophysiology and improve symptoms associated with HCM. Mavacamten is a first-in-class inhibitor of cardiac-specific myosin investigated for the treatment of HCM.5-7 It reduces the number of myosin–actin cross-bridges and thus decreases excessive contractility characteristic of HCM.
METHODS
The MAVERICK-HCM study8 explored the safety and efficacy of mavacamten in nHCM. It was a multicentre, double-blind, placebo-controlled, dose-ranging Phase II study in adults with symptomatic nHCM (New York Heart Association [NYHA] Functional Class II/III), LV ejection fraction (LVEF) ≥55%, and N-terminal pro b-type natriuretic peptide (NT-proBNP) ≥300 pg/mL. Participants were randomised 1:1:1 to mavacamten at a pharmacokinetic-adjusted dose that targeted plasma levels of 200 or 500 ng/mL or placebo for 16 weeks, followed by an 8-week washout. The initial dose of mavacamten was 5 mg qd with one dose titration (to 2.5, 10.0, or 15.0 mg) at Week 6.
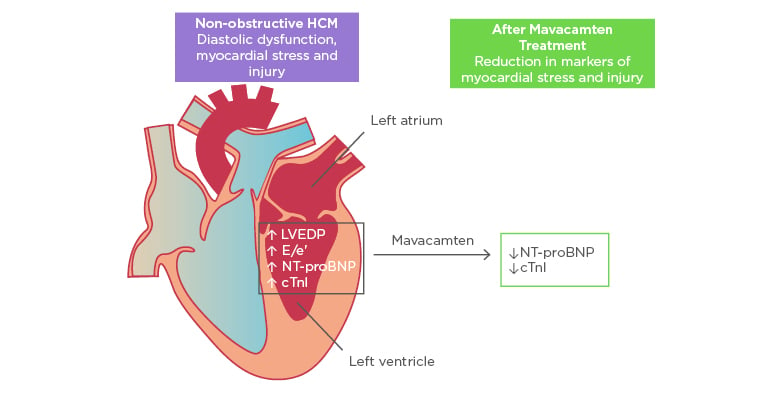
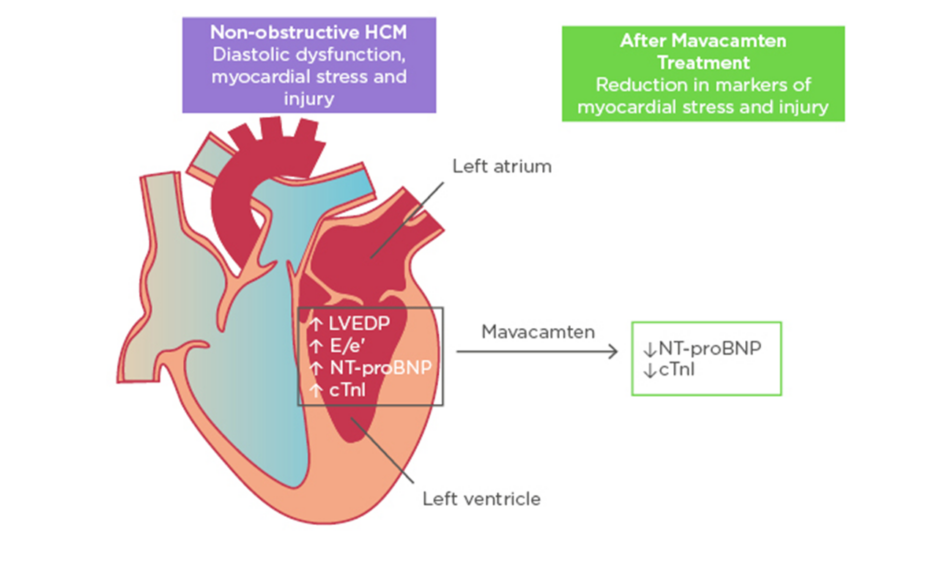
Figure 1: Improvement in biomarkers of cardiac stress and injury with mavacamten treatment.
cTnI: cardiac troponin I; E/e’: early diastolic transmitral flow velocity/early diastolic mitral annular velocity; HCM: hypertrophic cardiomyopathy; LVEDP: left ventricular end-diastolic pressure; NT-proBNP: N-terminal pro B-type natriuretic peptide.
Adapted with permission from Ho et al.11
The primary objective was to evaluate the safety and tolerability of mavacamten. Exploratory efficacy objectives included NT-proBNP and cardiac troponin I (cTnI) level changes from baseline to Week 16, as well as a composite functional endpoint that assessed the impact of mavacamten on peak oxygen consumption (pVO2) and NYHA Functional Class. The exploratory composite functional endpoint mirrored the primary endpoint in the ongoing Phase III EXPLORER-HCM trial in obstructive HCM.9,10 The trial aimed to achieve the following at Week 16 compared to baseline: 1) an improvement of at least 1.5 mL/kg/min in pVO2 and a reduction of 1 or more NYHA Functional Class; or 2) an improvement of 3.0 mL/kg/min or more in pVO2 with no worsening in NYHA Functional Class.
RESULTS
In total, 59 participants were randomised (19/21/19 to 200 ng/mL, 500 ng/mL, and placebo, respectively). Baseline characteristics were similar across treatment groups with a mean age of approximately 54 years, a 58% female predominance, and most patients exhibiting NYHA Functional Class II, although a larger proportion of participants with NYHA Functional Class III were randomised to placebo (31.6% [placebo] versus 17.5% [pooled mavacamten]).
Mavacamten was generally well-tolerated by most participants. Serious adverse events occurred in 10% of participants on mavacamten and 21% on placebo. Most serious adverse events were cardiovascular, including a similar incidence of recurrent atrial fibrillation and atrial flutter in mavacamten and placebo arms. In this dose-ranging, pharmacokinetic-driven study, there were five participants with a documented LVEF ≤45% who met prespecified stopping criteria (the nadir LVEF ranged from 38 to 45%). In all five participants, LVEF recovered towards baseline after 4–12 weeks.
In exploratory efficacy analyses, there were significant reductions in geometric means of NT-proBNP and cTnI, both biomarkers of myocardial wall stress and injury. NT-proBNP geometric mean decreased by 53% in the pooled mavacamten group versus 1% in placebo (p=0.0005). cTnI geometric mean decreased by 34% in the pooled mavacamten group versus a 4% increase in placebo (p=0.009). There was no clear difference observed in the proportion of participants achieving the composite functional endpoint in the intention-to-treat group. However, subgroup analysis of participants with more severe disease expression defined by elevated cTnI (>99th percentile) or early diastolic transmitral flow velocity/early diastolic mitral annular velocity (E/e’)average >14 at baseline (21 participants on mavacamten and 12 participants on placebo) showed that 33% of mavacamten-treated participants met the composite functional endpoint versus none receiving placebo (p=0.03).
CONCLUSION
In summary, mavacamten, a novel myosin inhibitor, was well-tolerated in most participants with symptomatic nHCM. Furthermore, treatment was associated with a significant reduction in NT-proBNP and cTnI suggesting physiological benefits, and patients with more severe disease expression may benefit most from therapy (Figure 1). These results set the stage for future, larger scale studies of mavacamten in nHCM and potentially in HFpEF.