Chairperson: Carolyn Lam1
Speakers: Carolyn Lam,1 Burkert Pieske,2 Justin Ezekowitz,3 Javed Butler4
1. National Heart Centre, Singapore, Singapore
2. Charité University of Medicine, Berlin, Germany
3. University of Alberta, Edmonton, Canada
4. University of Mississippi, Oxford, Mississippi, USA
Disclosure: Prof Lam has received research support and/or served as a consultant or on the advisory board/steering committee/executive committee for Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc, EKo.ai, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group, Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma, and WebMD Global LLC; and is cofounder and non-executive director of EKo.ai. Prof Pieske has acted as a speaker, advisor, or steering committee member for Novartis, Bayer, Merck, Servier, AstraZeneca, Bristol Myers Squibb, Daiichi-Sankyo, and Medscape. Prof Ezekowitz is a member of the VICTORIA executive committee, a trial funded by Bayer and Merck Shape & Dohme. Prof Butler has received research support and/or served as a consultant or on the advisory board/steering committee/executive committee for Abbott, Adrenomed, Array, Amgen, Applied Therapeutics, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Corvia, Cardior, CVRx, Eli Lilly and company, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave Ltd, and Vifor Pharma.
Acknowledgements: Writing assistance was provided by Helen Boreham, HB Medical (UK) Ltd, Leeds, UK.
Support: The publication of this article was funded by Bayer. The views and opinions expressed are those of the presenters. Content was reviewed by Bayer for medical accuracy.
Citation: EMJ Cardiol. 2020;8[1]:26-33.
Meeting Summary
Despite clinical advances in the management of heart failure with reduced ejection fraction (HFrEF), new treatment strategies are still urgently needed for patients with symptomatic chronic HF who have experienced a previous worsening HF event. These patients remain at risk of recurrent worsening HF events despite optimal, guideline-directed medical and device therapy. During this symposium, leading cardiology experts explored therapeutic advances in patients with HFrEF who have had a previous worsening HF event, focussing on the novel cyclic guanosine monophosphate (cGMP) pathway stimulator vericiguat, as well as results from the landmark VICTORIA trial.
Introduction from the Chair
Professor Carolyn Lam
European and USA treatment guidelines are aligned in their standard recommendation of therapies for symptomatic HFrEF.1,2 First-line treatment is centred on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers plus β-blockers, with diuretics as needed. Next steps in the treatment pathway include adding mineralocorticoid receptor antagonists, replacing an angiotensin-converting enzyme inhibitor with an angiotensin receptor neprilysin inhibitor, or adding ivabradine in patients with sinus rhythm (heart rate ≥70 beats per minute).1,2 However, Prof Lam stressed that an important residual risk remains in many patients with HFrEF despite the guidelines-directed use of these available HF medications.
Landmark trials have highlighted the successes that have been achieved with the current armoury of HFrEF therapies.3-7 Yet even in the most recent of these studies, DAPA-HF, residual risk of cardiovascular (CV) death remained at 10% in the treated arm over 18 months’ follow-up.7 This risk is accentuated following a HF event. Real-world data from the PINNACLE registry showed that 56% of patients were rehospitalised within 30 days of a worsening HF event, and the number of HF-related hospitalisations increased over time.8
Therefore, although baseline risk in patients with HFrEF can be successfully lowered with standard guideline-directed therapies, it is important to recognise that a worsening HF event or hospitalisation is a key sign of increasing risk. These patients typically follow a worsening disease trajectory, with risk of further hospitalisations eventually culminating in advanced HF.9 Prof Lam posed the important clinical question: can we bend this curve in worsening HF, delay the progression of worsening HF events, and improve outcomes for patients with HFrEF?
Stimulating sGC: VICTORIA Trial Reveals Improved Outcomes in Patients with HFrEF
Professor Burkert Pieske
HFrEF is a complex systemic disorder in which a number of abnormal and dysregulated pathways interact, leading to progressive cardiac remodelling and repeat decompensations. Although a number of these pathways are already medically addressed with existing treatment modalities, important therapeutic targets remain, including the nitric oxide (NO)–soluble guanylate cyclase (sGC)–cGMP pathway.1,10-18
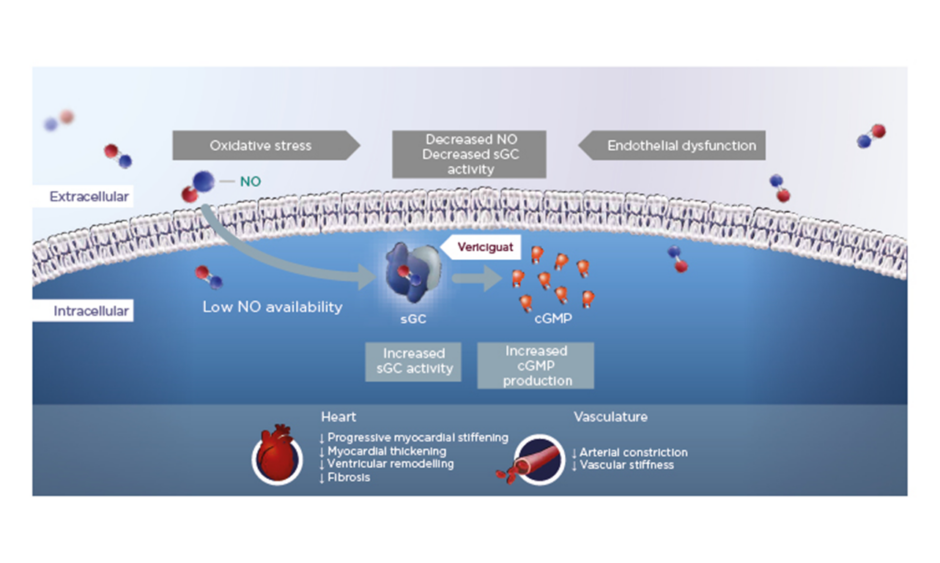
In HFrEF, increased oxidative stress reduces NO bioavailability, which in turn leads to reduced activity of the important enzyme sGC and lower levels of cGMP. Vericiguat is a once-daily oral therapy that stimulates sGC, thereby restoring intracellular cGMP levels and leading to improved myocardial and vascular function in HF (Figure 1).12,15,19-24
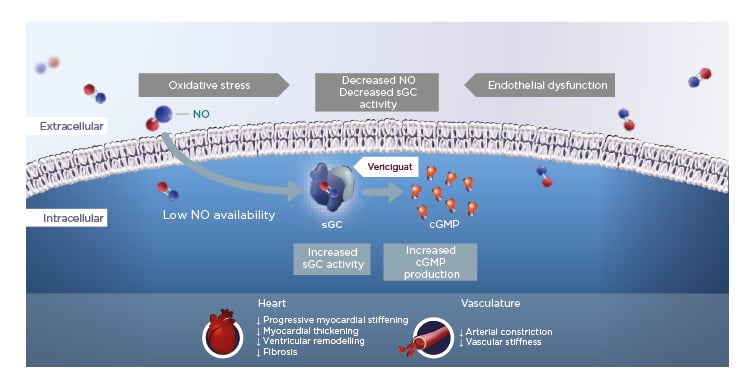
Figure 1: Vericiguat increases soluble guanylate cyclase activity to improve myocardial and vascular function.12,15,19-24
cGMP: cyclic guanosine monophosphate; NO: nitric oxide; sGC: soluble guanylate cyclase.
VICTORIA was an international, Phase III, randomised, parallel-group, placebo-controlled, double-blind, event-driven, outcome trial of vericiguat in patients with symptomatic chronic HF who had a previous worsening HF event despite currently available HF therapies.22,25 A total of 5,050 patients were randomised 1:1 to vericiguat or placebo, with the target dose of 10 mg once daily achieved in 89.2% and 91.4% of patients, respectively, after 12 months. The primary endpoint was time to first occurrence of the composite of CV death and first HF hospitalisation.22,25
Eligibility criteria for VICTORIA included symptomatic chronic HF, New York Heart Association (NYHA) Class II–IV, and left ventricular ejection fraction (LVEF) <45% on optimal background HF therapies plus a worsening HF event, defined as recent HF decompensation (HF hospitalisation or intravenous [IV] diuretic use) and elevated natriuretic peptides.22,25 Prof Pieske described VICTORIA patients as ‘high risk’, with 84% having experienced an index HF hospitalisation within 6 months. Otherwise, VICTORIA included a typical advanced HFrEF patient population: elderly (mean age 67 years) and predominantly male (76%).
Patients were overtly symptomatic (NYHA Class II: approximately 60%; Class III: 40%), with mean LVEF of 29% at screening.25 Around 60% of patients were on three baseline standard of care therapies, including approximately 15% on sacubitril/valsartan.25
After 10.8 months’ follow-up, vericiguat significantly reduced the cumulative incidence rate of time to CV death or first HF hospitalisation versus placebo (p=0.02; hazard ratio [HR]: 0.90; 95% confidence interval [CI]: 0.82–0.98), meeting VICTORIA’s primary endpoint (Figure 2).25 The absolute risk reduction with vericiguat was 4.2% per year, translating to an annual number needed to treat of 24.25 For the individual components of the primary endpoint, vericiguat achieved a 7% relative risk reduction in time to CV death and 10% reduction in time to HF hospitalisation, confirming its beneficial impact in this high-risk patient population.25,26
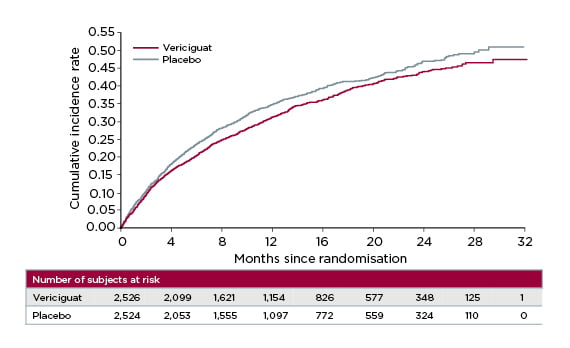
Figure 2: VICTORIA trial primary endpoint: time to cardiovascular death or first heart failure hospitalisation.25
In terms of secondary outcom–s, vericiguat significantly reduced total HF hospitalisations (p=0.02; HR: 0.91; CI: 0.84–0.99) and significantly lowered the composite of first HF hospitalisations and all-cause mortality (p=0.02; HR: 0.90; CI: 0.83–0.98) compared to placebo.25 Prespecified subgroup analysis of the primary endpoint showed a consistent benefit of vericiguat treatment across the majority of subgroups.25 Younger patients tended to benefit slightly more from vericiguat therapy than older subjects in the study.25 Prof Pieske also highlighted the impact of N-terminal pro-brain natriuretic peptide (NT-proBNP) levels at baseline as an interesting signal requiring further evaluation. Patients with baseline NT-proBNP levels in the lower three quartiles showed a more pronounced benefit from vericiguat compared to the highest quartile (>5,314 pg/mL).25
Overall, vericiguat proved to be well tolerated in the VICTORIA trial, with an overall adverse event (AE) profile and incidence of serious AE comparable to that of placebo.25 Patients on vericiguat were slightly more likely to develop mild anaemia versus placebo (7.6% versus 5.7%), but there was no interaction with electrolyte balance.25 Looking at AE of clinical interest, there were no significant differences in the rates of symptomatic hypotension or syncope between vericiguat and placebo.25
Although minor declines in systolic blood pressure were noted early in the uptitration phase, no further clinically relevant reductions in blood pressure were observed throughout the remainder of the study.25 There were no decreases in estimated glomerular filtration rate (eGFR) with vericiguat therapy, despite use in patients with baseline eGFR as low as 15 mL/min/1.73 m2.25
In summary, vericiguat marks a potential advance in the treatment of HFrHF, enhancing the cGMP pathway to improve both myocardial and vascular function. Prof Pieske described efficacy findings from the VICTORIA trial as ‘clinically relevant’, with vericiguat significantly reducing the annualised risk of the VICTORIA composite outcome of time to HF hospitalisation or CV death by 4.2%.25
New Insights from a Deep Dive into VICTORIA Data and Its Potential Impacts
Professor Justin Ezekowitz
In the VICTORIA trial, treatment heterogeneity was observed in baseline NT-proBNP levels (which were prespecified into quartiles); hence, continuous analysis was carried out to further explore the relationship between NT-proBNP and HF event outcomes. Results from this analysis were discussed in a deep dive by Prof Ezekowitz. Overall, the treatment effect of vericiguat on the primary outcome proved consistent across most of the 13 prespecified subgroups analysed in the VICTORIA trial, with a clear benefit favouring vericiguat over placebo irrespective of sex, geographical region, index event, sacubitril/valsartan use, NYHA class, renal function, or ejection fraction.25 A weak interaction with age was identified that requires further interaction.25
Despite the overall homogeneity of the VICTORIA trial findings, an interaction was observed between treatment and the primary outcome according to NT-proBNP levels.25 NT-proBNP is known to be a strong marker of prognosis in patients with HFrEF.27,28 Data from the Swedish registry have highlighted the clear link between higher NT-proBNP levels and an increasing risk of CV events in patients with HFrEF.28 NT-proBNF is also used as an inclusion criteria for clinical trials and is linked to treatment efficacy, with evidence of an association between treatment-related changes in natriuretic peptides and clinical effects such as HF hospitalisation.27
In the VICTORIA trial, continuous analysis across the spectrum of NT-proBNP demonstrated that the treatment effect of vericiguat was maintained at levels up to 8,000 pg/mL, representing 86% of the study population (Figure 3).29 Prof Ezekowitz explained that NT-proBNP levels >10,000 pg/mL are uncommon in both clinical practice and the clinical trial setting. The HR for vericiguat versus placebo for the primary composite endpoint was 0.85 in patients with NT-proBNP levels ≤8,000 pg/mL.29 This treatment effect of vericiguat was further amplified in patients with NT-proBNP levels ≤4,000 pg/mL, accounting for 65% of the VICTORIA population, with a HR for the primary composite outcome of 0.77.29 Looking at the individual components of the primary endpoint, the treatment effect of vericiguat in patients with NT-proBNP levels ≤8,000 pg/mL was found to extend to both CV death and HF hospitalisation, with similar HR for both.29
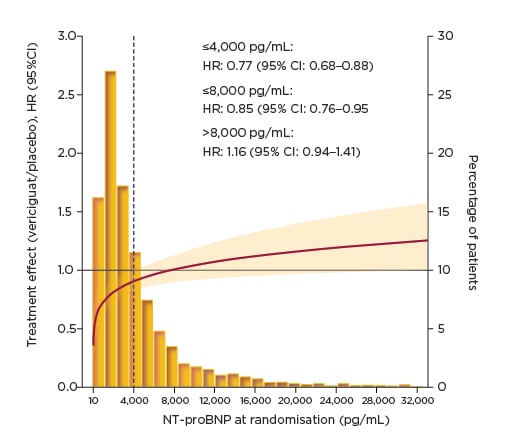
Figure 3: VICTORIA primary composite outcome: clinical outcomes by N-terminal pro-brain natriuretic peptide at randomisation.29
CI: confidence interval; HR: hazard ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
When carrying out post hoc analysis such as this, it is important to employ robust statistical methods and consider potential unmeasured confounders. Prof Ezekowitz explained that values from this NT-proBNP subgroup analysis were adjusted with the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score and also proved consistent when validated with an internally-derived risk score from the VICTORIA trial, indicating a ‘robust’ data set.29
Putting the VICTORIA Trial into Perspective with Contemporary HFrEF Clinical Trials
Professor Javed Butler
The important question of how to compare different HFrEF clinical trials to make therapeutic decisions based on data was discussed by Prof Butler. Although similar in size, scope, and primary endpoint, VICTORIA was fundamentally different to contemporary HFrEF trials in its patient population. In addition to minor differences in parameters such as blood pressure and NT-proBNP, VICTORIA enrolled a broader population in terms of ejection fraction (<45%) compared to DAPA-HF (≤40%) and PARDIGM-HF (≤35%), and importantly included patients with an eGFR cut-off as low as 15 mL/min/1.73 m2.6,7,22,25,30,31 Prof Butler noted that there is a clinical need to gather more data in this often overlooked renally-impaired patient population.
The VICTORIA trial stood out against other HFrEF trials because of its requirement, by design, for patients not only to have chronic symptomatic HF but also a worsening HF event.22,32,33 The rationale for selecting such patients was to focus on a population with substantial unmet need who require new treatment strategies. Prof Butler emphasised that patients with worsening HF despite optimal use of guideline-directed medical therapy are at substantially increased risk of poor prognosis. Aggressive treatment, both with known therapies if there are gaps and novel therapies if available, is therefore vital to move these patients back to the ‘residual’ risk category and prevent progression to advanced HF. Evidence indicates that within 1.5 years of a new HFrEF diagnosis, around 17% of patients will develop worsening HF.8 The 2-year mortality rate currently stands at approximately 23% in patients with symptomatic chronic HF who have experienced a previous worsening HF event.8
Examining study characteristics for the contemporary HFrEF trials at baseline reveals that inclusion of the ‘worsening HF event’ criteria in VICTORIA had a significant impact on the resulting patient population. Median NT-proBNP levels were substantially higher in VICTORIA patients (2,816 pg/mL) compared to both DAPA-HF (1,437 pg/mL) and PARADIGM-HF (1,608 pg/mL).6,7,25,30,34,35 VICTORIA also enrolled a greater proportion of patients with NYHA Class III/IV at baseline: 41% versus 32% and 25%, respectively. Compared to a minority of the patients in DAPA-HF (16%) and PARADIGM-HF (31%), the vast majority of VICTORIA patients (84%) had experienced HF hospitalisation within 6 months.6,7,25,30,34,35 The remaining 16% of VICTORIA patients had previously undergone IV diuretic treatment, equating to an overall study population of whom 100% had experienced a recent HF event.25
However, the conclusive evidence came from the outcomes, explained Prof Butler. In VICTORIA, the event rate for the primary endpoint in the comparator arm was 37.8 per 100 patient-years (PY), which was more than double that of the other two trials (DAPA-HF: 15.6 per 100 PY; PARADIGM_HF: 13.2 per 100 PY).6,7,22,25,26,30,34,35 Similarly, event rates for HF hospitalisation in the comparator arm were more than three times higher in VICTORIA (29.1 per 100 PY) compared to DAPA-HF (9.8 per 100 PY) and PARADIGM-HF (7.7 per 100 PY), and event rates for CV death were doubled (13.9 per 100 PY versus 7.9 per 100 PY and 7.5 per 100 PY, respectively).6,7,22,25,26,30,34,35
Understanding the high-risk nature of the VICTORIA patients helps to put the trial results into clinical perspective. Relative risk reduction for the primary endpoint in the VICTORIA trial was approximately 10%, but because of the high-risk nature of the patient population, the absolute risk reduction was 4.2%; this translated to an annual number needed to treat of only 24 to achieve the composite endpoint benefit.25,26 This reflects the high event rate for the primary endpoint and its components in the comparator arm despite guidelines-directed medical therapy. All events for the primary endpoint were collected within just 10 months of follow-up.25,26
Other ongoing trials in the HFrEF arena include the EMPEROR-Reduced study of empagliflozin (the results from this study were not available at the time the symposium took place) and the GALACTIC-HF trial of omecamtiv mecarbil.36-39 These are large trials with primary endpoints comparable to VICTORIA. However, VICTORIA enrolled a wider group of patients in terms of LVEF inclusion criteria (<45%) than both EMPEROR-Reduced (≤40%) and GALACTIC-HF (≤35%).22,25,31,36-39 While EMPEROR-Reduced and GALACTIC-HF employed lower eGFR cut-offs (≥20 mL/min/1.73 m2) compared to previous HFrEF trials, VICTORIA remains the lowest for renal function requirements at >15 mL/min/1.73 m2.22,25,31,36-39 In terms of prior HF events, EMPEROR-Reduced inclusion criteria included chronic HF for ≥3 months with no current acute decompensated HF requiring IV diuretics, vasodilators, inotropic agents, or mechanical support within 1 week of screening nor during the screening period prior to randomisation.36,37 GALACTIC-HF patients were required to have chronic HF with current HF hospitalisation or ≤1 year of screening.38,39
Prof Butler concluded that, although EMPEROR-Reduced and GALACTIC-HF include some high-risk patients, VICTORIA remains the only trial to focus primarily on the high-risk group of patients with HFrEF with a previous worsening HF and is the first study to show therapeutic benefit in this important population.