Abstract
Nail lichen planus (NLP) is a chronic inflammatory disorder that can lead to irreversible damage, such as pterygium and anonychia. While NLP may initially present with subtle nail matrix changes, its unpredictable course and potential for permanent dystrophy highlight the need for early recognition and timely intervention. However, the evidence guiding NLP management remains limited, with most recommendations based on case reports and retrospective studies. This review summarises the current understanding of NLP, including recent insights into its pathogenesis, epidemiology, clinical features, diagnosis, and therapeutic strategies. Topical treatments alone have limited efficacy due to poor drug penetration and are not recommended as monotherapy. Intralesional corticosteroids remain the mainstay of treatment and should be considered early. Systemic corticosteroids, either via intramuscular injection or oral administration, can be considered in severe NLP, but long-term use may be limited by relapse and adverse effects. Oral retinoids, particularly alitretinoin, offer moderate benefit in mild or early-stage disease. Immunosuppressive agents may be reserved for refractory cases. Notably, while most recent findings regarding the JAK-signal transducer and activator of transcription pathway have emerged from studies on cutaneous or mucosal lichen planus, early reports of successful treatment in NLP using JAK inhibitors offer promising new insights for this challenging condition.
Key Points
1. Nail lichen planus (NLP) is a rare but potentially disfiguring nail disorder that can lead to permanent nail damage. This review provides a comprehensive synthesis of current knowledge on NLP, addressing critical gaps in diagnosis, classification, and treatment strategies, based on recent clinical insights and expert consensus.
2. This review outlines the clinical, histopathological, and immunopathogenic features of NLP and introduces a newly proposed clinical severity grading system (Typical Nail Lichen Planus Severity Index [tNLPSI]). It also compares available treatment options, from topical therapies to systemic immunosuppressants and JAK inhibitors, emphasising both practical application and emerging trends.
3. Timely recognition and intervention are crucial in preventing permanent nail damage in NLP. Intralesional corticosteroids remain a cornerstone of early treatment, while systemic options, such as intramuscular or oral corticosteroids, may be effective in more extensive disease, but are limited by relapse and adverse events. Retinoids offer modest benefit in milder cases. Encouragingly, emerging evidence from lichen planus research suggests that JAK inhibitors may represent a promising therapeutic option in difficult-to-treat NLP.
INTRODUCTION
Lichen planus (LP) is a chronic, immune-mediated inflammatory disorder that affects the skin, mucous membranes, hair, and nails.1 Among these, nail lichen planus (NLP) is an uncommon but potentially destructive variant that may lead to permanent nail deformities, such as pterygium and anonychia.2 NLP can occur in isolation or alongside other LP subtypes, and its clinical spectrum ranges from subtle longitudinal ridging to complete nail plate loss, often mimicking other nail disorders and resulting in delayed diagnosis.3 Although NLP has gained increasing recognition in recent years, high-quality data remain limited. Most literature consists of small cohorts, case reports, or expert consensus, with limited representation across ethnicities and long-term follow-up.2 Diagnostic challenges persist due to the non-specific clinical features of NLP, which may overlap with other dystrophic nail conditions.4 Despite a range of available treatments, from topical corticosteroids to systemic immunosuppressants, managing NLP remains challenging due to its recurrent nature.2 Recent developments, including insights into the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway and the emerging use of JAK inhibitors, offer promising new therapeutic avenues for the management of NLP.5 This review aims to summarise the current understanding of NLP, encompassing its pathogenesis, epidemiology, clinical and histopathological features, diagnostic approaches, and evolving therapeutic strategies, to guide clinicians and inform future research directions.
METHODS
A comprehensive literature search was conducted to identify peer-reviewed articles on LP, with a specific focus on isolated NLP. Searches were performed using the PubMed/MEDLINE and Web of Science databases for articles published up to January 2025. The following search terms were used alone or in combination: “lichen planus,” “nail lichen planus,” “isolated nail lichen planus,” “onycholichen planus,” and “nail dystrophy.” Reference lists from key articles and relevant reviews were also manually screened to identify additional studies. Only studies published in English were included. The review aimed to synthesise the current evidence base and identify recent advancements in understanding and managing NLP.
PATHOGENESIS
LP, including NLP, is considered a multifactorial inflammatory disorder, involving a complex interplay of genetic susceptibility, environmental triggers, and immune dysregulation.1 Genetic predisposition has been proposed based on familial cases and certain human leukocyte antigen (HLA) associations, such as HLA-A3, HLA-A5, HLA-A28, HLA-B8, HLA-B16, HLA-Bw35, HLA-B7, HLA-B18, HLA-Aw19, and HLA-Cw8, though specific genes remain undefined.6 Environmental factors, including drug exposure (e.g., antimalarial and antihypertensive agents),7 metal allergy (especially nickel and amalgam),8 and viral infections such as human papillomavirus,9 human herpesvirus,10 and hepatitis C virus,11 as well as, in rare cases, hepatitis B vaccination,11 have been reported as potential triggers in susceptible individuals. There is evidence that cell-mediated immune response plays a major role in the development of the disease.12 Current understanding indicates that the immunopathogenesis of LP is primarily driven by CD8+ cytotoxic T lymphocytes and CD45RO+ T cells, with their activity being modulated by the Th1 and IL-23/Th17 axes.12 Shao et al.13 demonstrated that CD8+ cytotoxic T cells induce keratinocyte apoptosis, possibly triggered by overexpression of major histocompatibility complex Class I molecules on keratinocytes, and interferon-γ plays a pivotal role in this process by upregulating major histocompatibility complex expression and activating the JAK2-STAT1 pathway. Inhibition of the JAK-STAT pathway has shown therapeutic efficacy in different subtypes of LP.5 Taken together, LP is increasingly understood as a complex immune-mediated condition involving coordinated actions of cytotoxic T cells, helper T cell subsets, cytokines, and innate immune elements.
EPIDEMIOLOGY
LP affects approximately 0.5–1.0% of the general population, with nail involvement reported in 10.0–15.0% of cases.14 NLP may occur in isolation or alongside cutaneous or mucosal LP, and although it can affect individuals of any age, it most commonly presents during the fifth and sixth decades of life.4 NLP appears to be slightly more prevalent in males, though some studies have shown no significant sex predilection.4,15 Adult patients are more frequently affected than children, in whom NLP remains relatively rare and often underdiagnosed.16 Fingernails are more frequently involved than toenails, with isolated toenail involvement being distinctly uncommon. Involvement may be asymmetric or symmetric, and while single-nail presentations do occur, multi-nail dystrophy is more typical in moderate-to-severe disease.
CLINICAL FEATURES
NLP presents with a broad spectrum of changes affecting the nail matrix, nail bed, and nail folds (Figure 1), with nail matrix involvement being the most predominant and diagnostically significant feature (Table 1).2,17 Common matrix-associated findings include longitudinal ridging, splitting, onychoschizia, trachyonychia, thinning of the nails, red or ‘mottled’ lunula, longitudinal melanonychia, and pterygium.15 Matrix-related changes were observed in 97.53% of patients in the authors’ unpublished cohort, consistent with prior reports of approximately 91.00% by Goettmann et al.,4 underscoring its central role in NLP pathogenesis. Among nail matrix involvement symptoms, longitudinal ridging (69.14%), splitting (48.15%), and nail plate thinning (43.21%) were some the most common findings in the authors’ cohort. Erythronychia, or red lunula, was observed in 29.63–31.30% of patients, which can be histopathologically correlated with distal nail matrix inflammation. Longitudinal melanonychia, while less frequent, is observed more often in individuals with skin of colour.18 Dorsal pterygium, defined as an irreversible alteration secondary to chronic inflammation-induced matrix scarring, was observed in 7.41–17.90% of cases. Interestingly, the occurrence of pterygium didn’t correlate with the duration of the disease.4 Changes in the nail bed may cause onycholysis, subungual hyperkeratosis, and splinter haemorrhages,15 which were documented in 29.63–43.30% of patients.4 Inflammation of the nail fold can result in periungual hyperkeratosis, erythematous lesions, and scaling of the proximal nail fold, which were identified in 17.28–18.00% of patients. Besides, an acute onset of pain may be indicative of bullous NLP.19
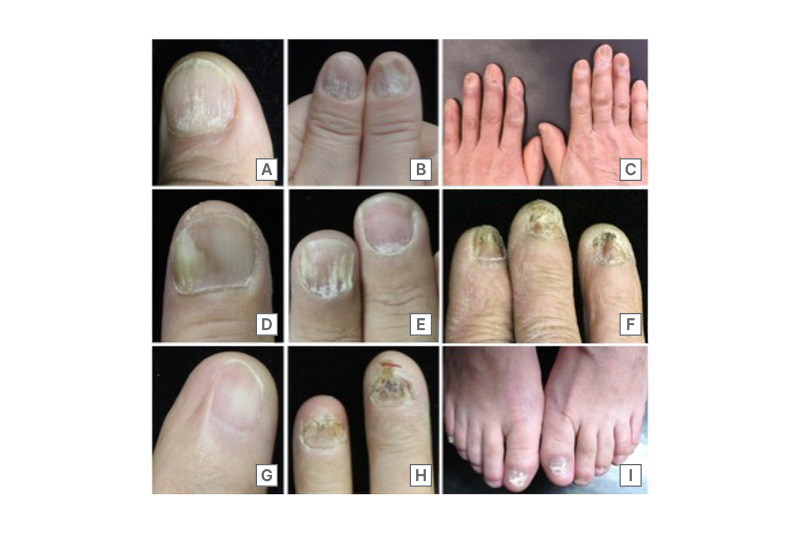
Figure 1: Clinical manifestations of nail lichen planus.
A) and B) Longitudinal ridging and splitting are among the most common features of matrix involvement in NLP. C) A representative case of typical NLP showing nail plate thinning, prominent longitudinal melanonychia, and periungual erythema with scaling. D) and E) Nail bed involvement characterised by distal onycholysis. F) Early stage of dorsal pterygium formation with adhesion of the proximal nail fold to the matrix, and partial loss of nail plate continuity. G) Fully developed dorsal pterygium presenting as a V-shaped extension of the proximal nail fold onto the nail bed, replacing the nail plate. H) and I) Atrophic NLP with severe nail plate thinning, scarring, and permanent anonychia, reflecting irreversible damage in advanced stages of the disease.
NLP: nail lichen planus.
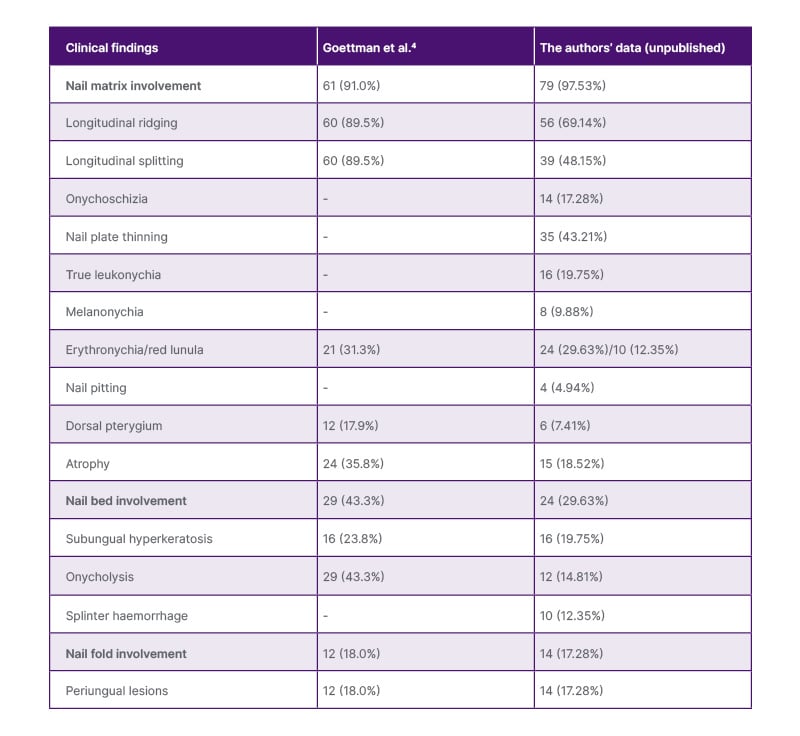
Table 1: The clinical features of nail lichen planus.
DIAGNOSIS
In most cases, the diagnosis of NLP is primarily clinical, guided by the recognition of characteristic patterns of nail matrix and nail bed changes, as the authors mentioned above. A detailed history and physical examination of other mucocutaneous sites should be performed to identify associated skin, oral, or genital LP, though NLP may occur in isolation. Dermoscopy serves as a useful adjunctive tool in identifying subtle features of NLP. For instance, NLP may manifest as linear discolouration of the nail bed, displaying alternating hues of blue, brown, red, or white while sparing the lunula. Although these subtle colour bands are often imperceptible to the naked eye, they can be readily detected using contact dermoscopy with ultrasound gel.20 Characteristic dermoscopic findings include longitudinal striae, red lunula, and trachyonychia-like surface roughness.21 However, dermoscopy remains underutilised in routine practice and may not replace histologic confirmation in uncertain presentations. Histopathological examination remains the gold standard for definitive diagnosis of NLP.22 The classic histologic presentations include hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis with band-like lymphocytic infiltrate at the dermoepidermal junction. Basal layer degeneration and Civatte bodies may also be observed.23 However, these histologic features may not be evident in more chronic NLP, which presents with nail matrix hypergranulosis and thin/atrophic nail unit epithelium.24 Longitudinal nail biopsies, particularly those that include both the nail matrix and ventral proximal nail fold, are recommended to ensure sufficient sampling. However, the risk of post-biopsy nail dystrophy necessitates careful patient selection and procedural planning.2
Differential diagnoses of NLP include onychomycosis, psoriasis, trachyonychia, alopecia areata-associated nail changes, and traumatic nail dystrophy.2 Fungal stains and cultures, along with histology, are crucial in distinguishing these entities. To support clinical assessment and standardised outcome measurement, the authors’ group recently proposed the Typical Nail Lichen Planus Severity Index (tNLPSI), a grading tool specifically designed for classical NLP.25 The tNLPSI evaluates both disease activity and irreversible damage by scoring key features of the nail matrix, nail bed, and nail fold. The tNLPSI activity scale was demonstrated to be consistent, reliable, reproducible, and feasible. It may prove to be a valuable tool in evaluating the treatment response in typical NLP clinical trials. Further validation and longitudinal testing are essential to establish the robustness and reliability of the tNLPSI scale.
TREATMENT
Due to the unpredictable nature of NLP progression, early therapeutic management is essential.26 Despite this clinical necessity, the current research landscape on NLP remains limited and fragmented. Most existing evidence stems from isolated case reports and retrospective studies (Table 2), with a notable lack of high-quality, prospective, and randomised controlled trials. Furthermore, the available research is characterised by considerable heterogeneity in treatment regimens, outcome measures, and follow-up durations, thereby reducing the comparability and generalisability of findings. This variability presents a significant barrier to the development of standardised, evidence-based treatment protocols.
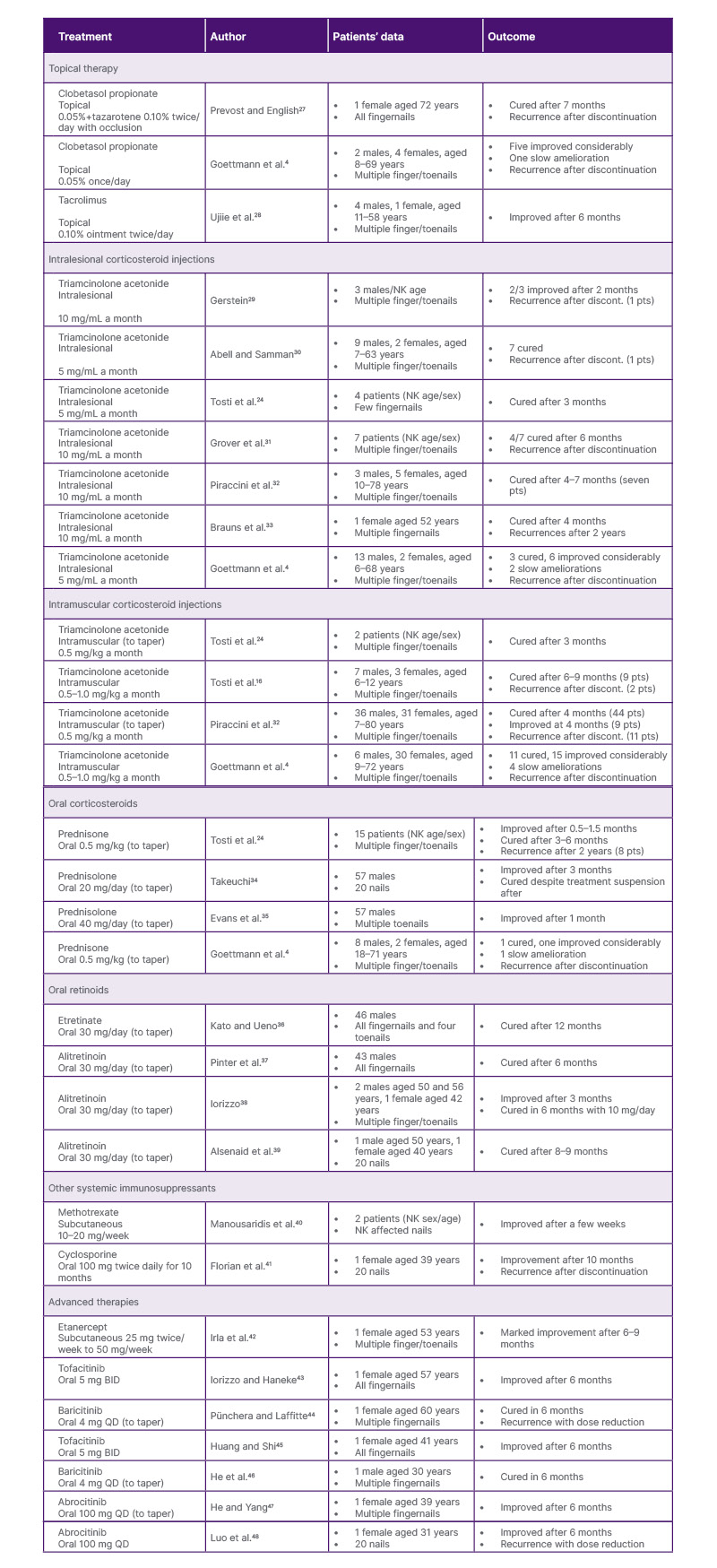
Table 2: Treatment options for nail lichen planus.
BID: twice daily; discont: discontinued; NK: not known; pts: patients; QD: once daily.
Based on the published literature and combined with the authors’ clinical observations, the authors found that topical therapies are largely ineffective for NLP, primarily due to the limited drug penetration. In contrast, localised interventions such as intralesional triamcinolone acetonide (TAC) injections, as well as systemic treatments including intramuscular (IM) and oral corticosteroids, have demonstrated more reliable therapeutic efficacy. However, these conventional approaches are frequently associated with high relapse rates following treatment cessation. Notably, emerging therapies, such as JAK inhibitors, represent a promising advancement, offering new hope for patients with refractory NLP by targeting key inflammatory pathways involved in disease pathogenesis.
Topical Therapy
Overall, topical treatments are not recommended as monotherapy therapy for NLP due to their limited drug penetration and the potential adverse effects associated with prolonged use. Both nail matrix and nail bed involvement in NLP typically respond poorly to topical agents, resulting in minimal clinical improvement. However, in cases where the periungual tissue is affected, particularly with erythema and scaling of the proximal nail fold, topical corticosteroids and topical calcineurin inhibitors, such as tacrolimus, may offer a clear therapeutic benefit by reducing local inflammation.
Intralesional Corticosteroid Injections
As outlined in the expert consensus by Iorizzo et al.,26 intralesional TAC injection is regarded as a first-line therapy for NLP. This approach allows for localised drug administration directly to the target tissue, offering enhanced therapeutic concentrations with reduced systemic exposure. While an optimal dosing regimen has not been standardised, TAC is most frequently used at concentrations of 2.5, 5.0, or 10.0 mg/mL. Injections are typically administered every 4–6 weeks, and clinical response is evaluated after a minimum of 4–6 months of continuous treatment. If significant improvement is observed, injection intervals may be extended to every 6–8 weeks. In contrast, a lack of response after six sessions should prompt reconsideration of the therapeutic approach.
However, patient discomfort during injections remains a common challenge. To enhance tolerability, various anaesthetic strategies can be employed. These include the pre-application of topical lidocaine cream 60 minutes before injection and the use of ethyl chloride spray.49 Distraction techniques, such as ‘talkesthesia’, vibratory devices, and percussive methods, have also proven helpful.49 For nail-bed involvement, digital nerve block anaesthesia is typically necessary.26 Although dermal atrophy is relatively rare, transient adverse effects such as pain, haematoma formation, and temporary distal numbness are more frequently reported.50 Despite these limitations, when administered with appropriate technique, intralesional injections remain a highly effective therapeutic option in the management of NLP.50
Intramuscular Corticosteroid Injections
IM triamcinolone offers a practical and effective therapeutic option for patients with NLP, particularly in cases involving multiple nails.51 TAC is the most commonly used agent, typically administered at a dose of 0.5–1.0 mg/kg every 4 weeks for a duration of at least 3–6 months. Doses of up to 1 mg/kg daily warrant consideration in patients with active or rapidly progressive disease.26 Iorizzo et al.26 recommend IM corticosteroids as an adjunct to intralesional administration in case of severe disease, especially when more than three nails are involved. Nonetheless, systemic therapy warrants consideration even with limited nail involvement when digitally critical nails (thumb, index, and middle fingers) exhibit clinically significant functional impairment.2 Besides, IM therapy is necessary and useful when intralesional injections are not feasible, whether due to anatomical challenges, physician inexperience, or patient discomfort.26 However, clinicians must remain mindful of potential contraindications, including uncontrolled diabetes, severe osteoporosis, active infections, peptic ulcer disease, and unstable psychiatric conditions.52
Oral Corticosteroids
Oral corticosteroids may also serve as an adjunctive option in the treatment of NLP. The indications for oral corticosteroid therapy are generally the same as those of IM use, particularly in cases involving extensive nail disease or functional impairment. While oral corticosteroids can achieve similar systemic effects, their long-term administration is often limited by a higher risk of cumulative side-effects, such as metabolic disturbances, mood changes, and gastrointestinal complications.52 Compared to oral administration, IM corticosteroid injections provide a more controlled and sustained drug release, potentially improving adherence and minimising daily fluctuations in serum drug levels. Iorizzo et al.26 have expressed a preference for IM corticosteroids over oral routes in NLP. Nonetheless, in certain cases, particularly when rapid dose adjustments are needed, or where oral administration allows for greater flexibility and physician control over tapering, oral corticosteroids may offer practical advantages over IM injections. Therefore, a carefully monitored course of oral corticosteroids remains a viable option, especially when IM delivery is not feasible or preferred by the patient.
Oral Retinoids
For patients who decline systemic corticosteroids, oral retinoids represent a viable alternative for NLP, particularly in mild-to-moderate cases. Acitretin, typically administered at 0.2–0.3 mg/kg/day, is known to be effective in patients with cutaneous LP,53 which functions by regulating keratinocyte proliferation and differentiation. Alitretinoin, used at a dose of 30 mg/day, offers several advantages over acitretin, including fewer side-effects, greater anti-inflammatory properties, and better regulation of keratinocyte differentiation and proliferation.54 In clinical practice, retinoid therapy is generally continued for several months, with gradual tapering considered upon achieving significant clinical remission. If no meaningful improvement is observed within 6 months, alternative treatments should be explored. Despite their efficacy, retinoids are associated with notable adverse effects, such as skin dryness, lip inflammation, hyperlipidaemia, hepatotoxicity, and teratogenicity, necessitating close patient monitoring.53
Other Systemic Immunosuppressants
Immunosuppressive agents such as azathioprine (100 mg/day), cyclosporine (3–5 mg/kg/day), and mycophenolate mofetil (1,000 mg twice daily) have been proposed as third-line options for NLP, particularly in patients who show inadequate response to first-line therapies.40 However, these agents are typically reserved for refractory cases due to their systemic immunosuppressive effects and limited supporting evidence. Clinical experience from both the authors’ team and the Iorizzo et al.26 group suggests that hydroxychloroquine and methotrexate, though commonly used in other inflammatory dermatoses, appear to lack therapeutic benefit in NLP and are therefore not recommended.
Advanced Therapies
Recent advances in targeted immunotherapy have introduced JAK inhibitors as promising agents in the management of NLP,43-48 particularly in patients with recalcitrant or isolated nail involvement unresponsive to conventional therapies. The rationale for their use is grounded in the immunopathogenesis of LP, in which interferon-γ-driven cytotoxic CD8+ T cell responses are believed to play a central role.12 These T cells target basal keratinocytes via the JAK-STAT signalling axis, triggering apoptosis and interface dermatitis.13 JAK inhibitors disrupt this signalling cascade, thereby reducing pro-inflammatory cytokine production, inhibiting T cell activation, and suppressing keratinocyte damage. Several case reports have supported the potential efficacy of JAK inhibitors in NLP. Baricitinib (a JAK1/2 inhibitor),44,46 abrocitinib (a selective JAK1 inhibitor),47 and tofacitinib (a pan JAK inhibitor)43 have been reported to induce marked improvements in NLP. In the authors’ own experience, abrocitinib monotherapy led to a significant resolution in a patient with isolated NLP, with a favourable safety profile. Compared to other JAK inhibitors, selective JAK1 inhibition may offer an advantage by minimising haematological and metabolic side-effects often seen with broader JAK blockade.47 While these initial findings are encouraging, they remain limited to anecdotal evidence. Larger prospective studies are urgently needed to validate efficacy, establish optimal dosing regimens, and ensure long-term safety, particularly given the potential for systemic immunomodulation.
CONCLUSION
NLP is a rare but potentially scarring inflammatory nail disorder with unpredictable progression. Early recognition and intervention are crucial to prevent irreversible damage. While evidence-based treatment strategies remain limited, intralesional and systemic corticosteroids are currently the most effective options. Retinoids and immunosuppressants may serve as alternatives in select cases. Emerging therapies, such as JAK inhibitors, show promising potential and warrant further investigation.