Seminar Summary
In this CME session at the 2025 Fall Clinical Dermatology Conference, held in Las Vegas, Nevada, USA, between October 23–26, 2025, leading experts in dermatology examined clinical evidence for the use of JAK inhibitors in the long-term management of alopecia areata (AA) and discussed strategies to optimize treatment of AA using these important new additions to the therapeutic armory. JAK inhibitors have revolutionized the treatment landscape in AA over recent years and three agents from this class are now approved: baricitinib, ritlecitinib, and deuroxolitinib. Using real-world case-based examples, experts highlighted key considerations in the management of patients with AA using JAK inhibitors, including treatment eligibility assessment, dose adjustment/titration, response monitoring, and patient counseling. The overarching aim of this educational seminar was to plug current knowledge gaps and enhance clinical decision-making in order to ensure JAK inhibitors are used in the safest and most effective way for the treatment of AA.
Exploring the Clinical Evidence for JAK Inhibitors
AA is an autoimmune condition in which the immune system attacks the hair follicles, presenting with transient, non-scarring hair loss. This disorder may eventually progress to total loss of scalp hair (alopecia totalis) and total loss of all body hair (alopecia universalis).1 The advent of JAK inhibitors has heralded a revolution in the treatment of AA, emphasized Adelaide Hebert, Chief of Pediatric Dermatology and Professor of Dermatology and Pediatrics at University of Texas McGovern Medical School, Houston, USA. “That we can actually give a patient a pill and have them regrow their hair…that was unimaginable not so many years ago,” she remarked.
Three oral JAK inhibitors are currently approved for AA and Hebert highlighted some key differences in their mechanism of action, dosing, indication, and required laboratory monitoring. Baricitinib (approved 2018) and the newest therapeutic option deuruxolitinib (approved 2024) are both JAK1/JAK2 inhibitors indicated for AA in adults aged 18+ years, while ritlecitinib (approved 2023) blocks JAK3 and TEC and is approved in patients aged 12+ years.2-5 In terms of dosing, deuruxolitinib (8 mg) is administered twice daily, while baricitinib (2 mg or 4 mg) and ritlecitinib (5 mg) are both once-daily options, “which simplifies the regimen to some degree,” noted Hebert.3-5 Laboratory monitoring (complete blood count) is recommended every 4–12 weeks for all JAK inhibitors. Baricitinib and deuruxolitinib also require a lipid profile and a CYP2C9 variant test is additionally recommended prior to starting deuruxolitinib. This test is easy to obtain, reassured Hebert, and is paid for by the manufacturer, not billed to the patient’s insurance. Unlike its fellow JAK inhibitors, deuruxolitinib does not require liver enzyme monitoring.2-5
Hebert went on to review some of the compelling clinical evidence supporting the use of JAK inhibitors in AA. In the THRIVE-AA1/AA2 clinical trials of deuruxolitinib, Hebert highlighted how patients continued to improve “well past the primary endpoint of 24 weeks.” Overall, 48.8% and 76.6% of patients achieved a Severity of Alopecia Tool (SALT) score ≤20 by Week 68 of treatment with deuruxolitinib assessed by last observation carried forward and as observed, respectively (Figure 1).6
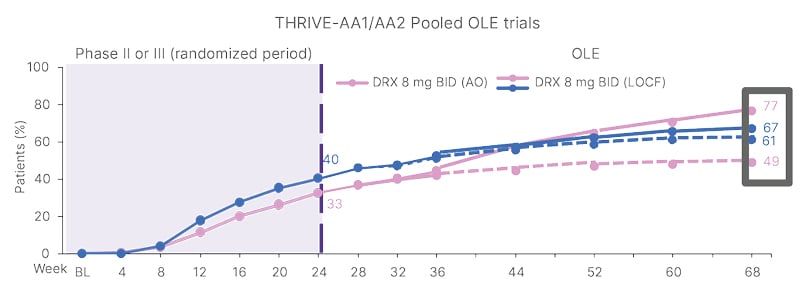
Figure 1: SALT score ≤20 with deuruxolitinib over 68 weeks.6
AO: as observed; BID: twice daily; BL: baseline; DRX: deuruxolitinib; LOCF: last observation carried forward; OLE: open- label extension; SALT: Severity of Alopecia Tool.
Long-term studies of baricitinib and ritlecitinib carried out over 3 years have also shown maintenance of hair growth in patients with AA treated with JAK inhibitors. In the BRAVE-AA studies of baricitinib, 83.6% and 89.1% of patients in the 2 mg and 4 mg dose groups, respectively, had SALT score ≤20 at Week 152.7 Similarly, the proportion of patients reaching SALT score ≤20 at Month 36 was 47.1% and 65.1% by last observation carried forward and as observed, respectively, in the ALLEGRO trials of ritlecitinib.8
Overall, these results show that patients with AA can achieve sustained success with JAK inhibitor treatment, Hebert observed.
Assessing Disease Severity and JAK Inhibitor Eligibility
Karan Lai, Director of Pediatric and Cosmetic Dermatology at Affiliated Dermatology, Scottsdale, Arizona, USA, went on to discuss the assessment of AA disease severity and patient eligibility for JAK inhibitor therapy. He highlighted a useful scale for classifying AA severity using the extent of scalp hair loss combined with other key factors. According to this scale, mild, moderate, and severe AA are indicated by scalp hair loss of ≤20%, 21–49%, and 50–100%, respectively.9
However, disease severity is defined by more than just scalp hair, stressed Lal, and additional factors can increase the severity of mild or moderate AA by one level if present.9 These include:
- Negative impact on psychosocial functioning resulting from severe AA
- Noticeable involvement of eyebrows or eyelashes
- Inadequate response after at least 6 months of treatment
- Diffuse (multifocal) positive hair pull consistent with rapidly progressive AA
Lal encouraged dermatologists to use this severity scale in their clinical charting and documentation, alongside standard SALT assessment, as it can help to open up access to JAK inhibitor therapy.
In terms of what defines alopecia severity for patients themselves, Lal pointed to results of a recent study emphasizing the impact of eyebrow hair loss in particular. In this study, patients’ satisfaction with theoretical treatment responses ranged from 32.8% with complete scalp hair growth and no brows to 90.4% for complete scalp hair and brows.10 Interestingly, patients were still 69% satisfied with complete brows and no scalp hair.10 “So we need to remember that the eyebrows are a very important area to assess and document,” stressed Lal.
Taking the Right Steps: Case Studies in Alopecia Areata Management
Experts then presented some patient case studies from their own clinical practice, illustrating the marked improvements in hair regrowth attained with the use of JAK inhibitors in the real-world setting.
Lal presented the case of Lucas, a 34-year-old male with relapsing AA on and off for 5 years and with progression towards a totalis phenotype. He had failed on a number of prior therapies, including oral minoxidil, topical and oral steroids, and methotrexate. Regular corticosteroid injections were incompatible with his work schedule, so the decision was made to initiate treatment with baricitinib. After 1 year of JAK inhibitor treatment, Lal confirmed that this patient has experienced full hair regrowth and remains on ongoing baricitinib therapy (at the lower 2 mg dose) with no adverse events.
Similarly impressive responses to JAK inhibitor therapy were seen in the case study of a 23-year-old male patient presented by Hebert. He had a history of AA spanning several years, including eyebrow alopecia, and had failed to respond to intralesional corticosteroids. After 24 weeks of ritlecitinib treatment, this patient had full scalp regrowth, and improvements were also seen in eyebrow and beard hair regrowth (Figure 2).
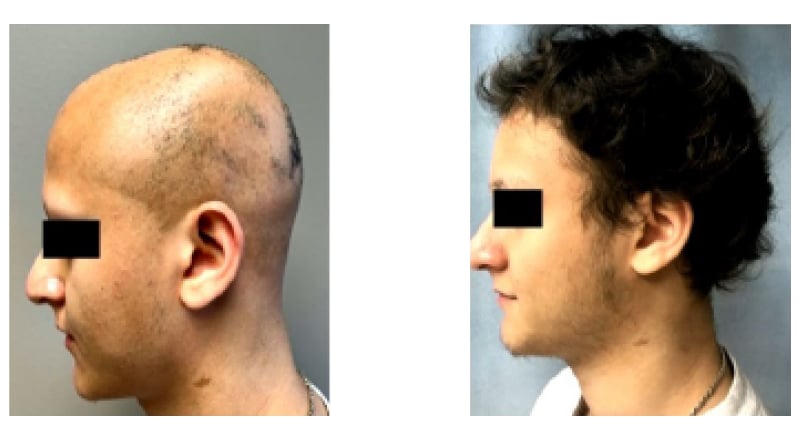
Figure 2: Case study: 23-year-old male patient with alopecia areata treated with JAK inhibitor.
“My experience is that we grow the scalp hair first, and then the brows and then the lashes,” observed Hebert. She also confirmed that, based on her own clinical experience, patients typically regrow hair which has similar characteristics (color, curliness, etc.) to that before the onset of AA.
Discussing the onset of the effect of JAK inhibitor treatment, experts noted that some patients may take longer to respond than others. Lal therefore suggested that it may be necessary to wait up to 1 or even 2 years in order to see the full effect of JAK inhibitor therapy.
Lal also pointed out that responders in real-world dermatology practice may look different to those in clinical trials. “Just because a patient didn’t meet the clinical criteria for the primary endpoint of a clinical trial, they could still have had a response,” he pointed out.
Optimizing Clinical Alopecia Areata Management with JAK Inhibitors
Addressing some of the challenges associated with the use of JAK inhibitors in clinical practice, Hebert acknowledged that the need for laboratory monitoring can prove burdensome for some patients, especially younger people with AA. She overcomes this obstacle by specifying the need for patients to get their laboratory monitoring done on the JAK inhibitor prescription itself. “If you don’t get your labs, you don’t get your refill…that’s the way that I inculcate that into my practice,” she explained. Hebert added that, in order to streamline the process, bloodwork can be done at patients’ own local testing facilities, with results then entered automatically into the electronic medical record for dermatologists to review in clinic.
Experts acknowledged that another task which can prove challenging in a busy clinic setting is calculating a SALT score, which is why alternative severity scales like the one presented previously are so useful. “I have my staff take pictures from all different angles, clipping the hair like we do clinical trials,” Lal explained. “These are just some little things to help if you’re having issues getting JAK inhibitor medication for your patients,” he added. Lal also noted that documenting beard alopecia can help facilitate eligibility for JAK inhibitor treatment, as the ability to grow a beard is an important priority for many male patients.
In the real-world setting, another question that patients often ask is what happens if JAK inhibitor treatment is stopped. Evidence from clinical trials of baricitinib that incorporated a withdrawal arm have indicated a rapid loss of response after JAK inhibitor cessation (Figure 3).11 Both experts therefore agreed that counseling patients on the reality of long-term, potentially life-long, JAK inhibitor therapy is important. “We have to explain to patients that if the medicine is withdrawn then gradual hair loss begins, usually after about 6 to 8 weeks,” Hebert elaborated.
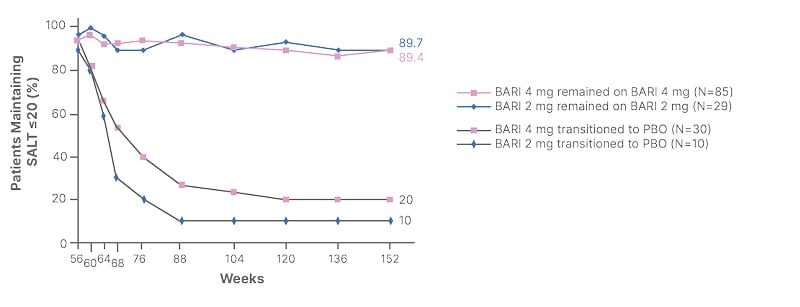
Figure 3: Loss of response after stopping baricitinib.11
BARI: baricitinib; PBO: placebo; SALT: Severity of Alopecia Tool.
Experts added that managing patients’ expectations and addressing misconceptions related to other aspects of JAK inhibitor therapy is also imperative. In particular, it is important to stress the need for long-term and consistent treatment adherence in order to optimize treatment response. “To manage patient expectations, don’t tell them that they will wake up tomorrow with a full head of hair as seen in the commercials,” cautioned Hebert.
Alongside the need for long-term JAK inhibitor treatment, it is also important to educate patients on the need for ongoing laboratory monitoring. Hebert advised that this monitoring should be performed “carefully and thoughtfully,” although she offered reassurances that abnormalities are encountered infrequently, particularly given the young and relatively healthy patient cohort affected by AA.
Finally, experts emphasized the need to set realistic treatment expectations for patients receiving JAK inhibitors, given that both the speed and extent of response can vary. Shorter disease duration and prior regrowth increase the likelihood of a favorable response, and better outcomes from JAK inhibitor treatment are observed in those with a disease duration less than 10 years. Nevertheless, experts agreed that patients with a long history of AA should not be precluded from treatment with a JAK inhibitor.
Conclusion
In summary, Lal reiterated that dermatologists now have access to three effective oral JAK inhibitors in their therapeutic toolkit for AA and alopecia universalis. Proper screening of patients and ongoing laboratory assessment are essential components of optimized clinical management. Ultimately, “this is a marathon, not a sprint,” Lal concluded, and with ongoing JAK inhibitor therapy, patients with AA can continue to improve and respond.