Meeting Summary
Clascoterone cream 1% is an androgen receptor inhibitor approved for the topical treatment of acne vulgaris (AV) in patients 12 years of age and older. This article summarizes data from a poster presentation on clascoterone cream 1% at the 2024 Fall Clinical Dermatology Conference, held in October 2024 in Las Vegas, USA. In this study, clascoterone cream 1% significantly reduced facial sebum production over 12 weeks of treatment in patients with mild-to-moderate acne. This marks the first clinical study to show an impact of clascoterone on sebum production and provides further evidence of its efficacy as a topical option for treating androgen-stimulated excess sebum production in male and female patients with AV.
Introduction
AV is a commonly occurring skin condition that currently affects around 50 million Americans, most frequently adolescents and young adults.1,2 Clascoterone cream 1% is approved for the topical treatment of AV on the basis of two randomized, vehicle-controlled Phase III trials and a 9-month extension study in which it demonstrated superior efficacy to vehicle creams.3-5 Clascoterone targets the androgen receptors that drive acne pathogenesis by stimulating overproduction of sebum, in turn creating a favorable environment for growth of the causative bacteria Cutibacterium acnes.6,7 Existing topical therapies for AV have not been shown to decrease sebum production, and systemic medications that act on sebaceous glands, such as isotretinoin or hormonal agents, are typically reserved for more severe cases or may not be suitable for both male and female patients.8,9 In vitro studies have already demonstrated that clascoterone binds with high affinity to the androgen receptor in sebocytes, inhibiting downstream androgen-stimulated gene transcription; however, its effect on sebum production in patients with AV has not been clinically demonstrated until now.7
Reduction in Facial Sebum Production Following Treatment with Clascoterone Cream 1% in Patients with Acne Vulgaris: 12-Week Interim Analysis10
Zoe Draelos from Dermatology Consulting Services, PLLC, USA, presented interim data through Week 12 from a 52-week, single-site clinical study designed to evaluate the effect of clascoterone cream 1% on facial sebum production and acne severity.
The primary efficacy endpoint was the reduction in casual sebum level measurements taken from the center of the patient’s forehead with a sebumeter. Casual sebum levels provide an objective measure of the lipid content present on the skin surface after remaining unwashed for several hours. Subjective measures of sebum production were also evaluated in this study, namely oily appearance, pore size, and facial shine, which were assessed by the investigator on a 5-point scale (0=none, 1=minimal, 2=mild, 3=moderate, 4=severe).
Additional efficacy endpoints included the Investigator’s Global Assessment (IGA) score (rated on a 5-point scale from 0 [clear] to 4 [severe]), inflammatory lesion count, and non-inflammatory lesion count. Tolerability assessments focused on the hallmark side effects of topical AV therapies:11 peeling, dryness, redness, and swelling (evaluated by the investigator) and stinging, burning, and itching (self-assessed by patients).
This study enrolled 40 patients with a mean age of 20.9 years, the majority of whom were female (60%) and White (63%). Just over half of patients (57.5%) began the trial with mild acne (IGA score: 2), while the remainder (42.5%) had moderate disease (IGA score: 3). The mean sebumeter reading was 115.9±50.5. Patients in this study applied clascoterone cream 1% twice daily to their entire face for 12 weeks, and endpoints were assessed at baseline and each fortnightly visit.
In the results from this interim analysis, clascoterone cream 1% demonstrated a significant improvement in sebumeter measurements, the primary outcome of the study, over 12 weeks of treatment. Mean (standard deviation) sebumeter readings decreased from 115.9 (±50.5) at baseline to 84.8 (±37.3) at Week 12, equating to an overall 27% reduction (P<0.001) in casual sebum levels (Figure 1). Statistically significant reductions from baseline in sebumeter measurements were observed from as early as Week 6 of treatment, with a mean reduction of 22% (P=0.002).
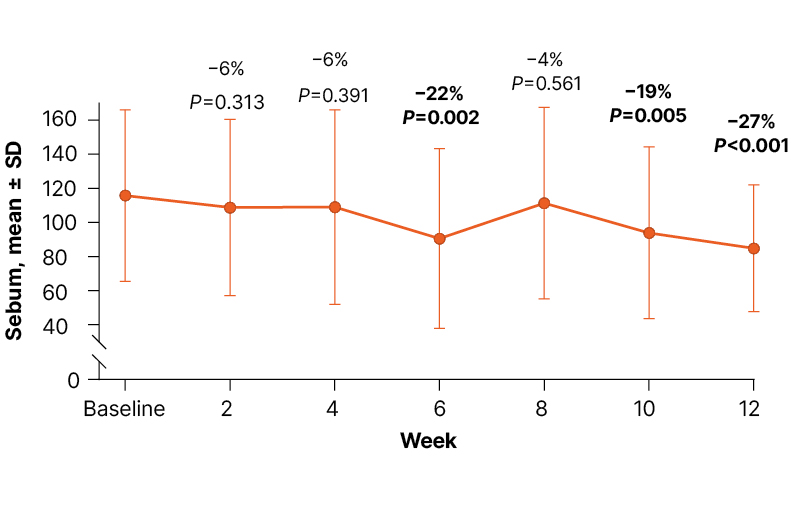
Figure 1: Sebumeter measurements by visit from baseline to Week 12.10
Data labels show the mean percent change relative to baseline.
Consistent with the objective decrease in sebum production, treatment with clascoterone cream 1% was also associated with statistically significant improvements in investigator-assessed facial characteristics, including oily appearance and facial shine by Week 4 and pore size by Week 6 (all P<0.01), with continued improvement through Week 12 (Figure 2). Overall, from baseline to Week 12, patients experienced a 40% improvement in facial appearance, a 23% improvement in pore appearance, and a 39% improvement in facial shine (all P<0.001).
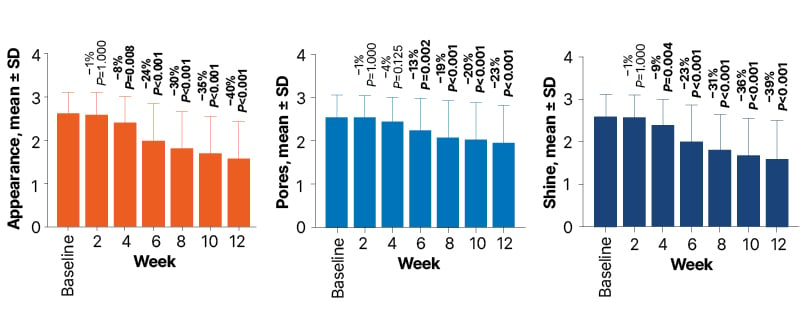
Figure 2: Facial appearance (A), pore size (B), and shine (C) by visit from baseline to Week 12.10
Data labels show the mean percent change relative to baseline.
Assessments were made on a 5-point scale from 0 (none) to 4 (severe).
In tandem with significant sebum reductions, patients treated with clascoterone cream 1% also showed significant improvements in their acne severity. Both inflammatory and non-inflammatory lesions were significantly reduced over 12 weeks of treatment, and the IGA score decreased significantly by 29%.
In terms of safety and tolerability, no adverse events or experiences had been reported with clascoterone cream 1% at the Week 12 interim analysis time point. Furthermore, no statistically significant changes in tolerability assessments, performed by either the investigator or patients themselves, were observed between baseline and Week 12 (all P<0.05).
Summary
This study provides initial clinical evidence of significant reductions in facial sebum production following 12 weeks of treatment with clascoterone cream 1% in both male and female patients with AV. Clascoterone cream 1% was well tolerated by all patients, with no report of any adverse reactions while using the treatment. Interim findings from this study replicate the improvements in acne severity seen in pivotal trials,4,5 and add new clinical evidence for reduction of sebum production as a key part of the mechanism of action of clascoterone for the treatment of AV.