Meeting Summary
Atopic dermatitis (AD) is associated with a high disease burden that impacts patients’ quality of life (QoL), particularly when specific difficult-to-treat or sensitive areas are involved, such as the head and neck (H&N). Patients with moderate-to-severe AD often require long-term treatment, and biologics are currently recommended as first-line systemic treatments for these patients. Tralokinumab is an approved biologic therapy with proven efficacy and safety in this population. New data on tralokinumab in AD were reported at conferences held in the latter half of 2024, including final study results from ECZTEND, demonstrating long-term efficacy and safety with up to 6 years of treatment; several analyses demonstrating efficacy in H&N and genital body regions; and a matching-adjusted indirect comparison (MAIC) of tralokinumab and lebrikizumab, demonstrating comparable maintenance of efficacy up to 52 weeks.
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, inflammatory skin condition, associated with a high disease burden that affects patients’ QoL.1,2 AD can affect multiple areas of the body, including the H&N region, and the genitals.3,4 However, not all affected areas have the same impact on patients’ QoL. For example, presentation of AD on the H&N, more than other body regions, is associated with social embarrassment, stigmatisation, and negative impact on patients’ QoL and mental health,5 and patients with AD in the genital area experience a higher disease burden and greater impairment of QoL compared to patients without genital involvement.4
Patients with moderate-to-severe AD often require long-term treatment, and biologics are currently recommended as first-line systemic treatment to prevent flares and maintain disease control.6,7 Tralokinumab, a monoclonal antibody that specifically targets IL-13, is indicated for the treatment of moderate-to-severe AD in patients ≥12 years of age in whom topical therapies are not advised or do not adequately control disease.8,9 Phase III trials (ECZTRA 1, 2, 3, and 6) have shown that tralokinumab is effective in this population,10-12 and an interim analysis of the open-label extension study ECZTEND demonstrated a favourable benefit–risk profile of tralokinumab in patients over the long-term.13
New tralokinumab data were reported at conferences held in the latter half of 2024, and a selection of these data are summarised below.
Robust Long-Term Efficacy of Tralokinumab and No New Safety Signals
At the Fall Clinical Dermatology Congress (FCDC) 2024, Blauvelt et al.14 presented final study results for ECZTEND, demonstrating the long-term efficacy and safety of tralokinumab in adults and adolescents with moderate-to-severe AD treated for up to 6 years.
The primary objective of ECZTEND was to assess the safety of tralokinumab from baseline to Week 268. Secondary objectives were to assess efficacy, including proportions of patients achieving Investigator’s Global Assessment score of 0 or 1 (IGA 0/1; clear/almost clear skin) or ≥75% improvement in Eczema Area and Severity Index (EASI-75) from parent trial baseline, and QoL measures up to Week 248.14
ECZTEND included patients who completed any of the nine parent trials (ECZTRA 1–8 and the TraSki investigator-initiated study). A total of 1,672 patients received tralokinumab 300 mg every other week, and concomitant use of topical corticosteroids (TCS) and/or calcineurin inhibitors was permitted. Median tralokinumab exposure was 2.6 years (4,466.2 patient years [PY]), and maximum exposure, including the parent trial period, was 6.1 years.14
Overall, the exposure-adjusted incidence rate (EAIR) of patients with at least one treatment-emergent adverse event (TEAE) was 114.3 per 100 PY. This figure was considerably lower than the EAIR observed in the initial 16-week treatment period of the parent trials (424.8 per 100 PY for tralokinumab; 475.3 per 100 PY for placebo). Over 5 years in ECZTEND, the most frequently reported TEAEs (≥5% of patients) were nasopharyngitis (22.2%), atopic dermatitis (21.4%), coronavirus infection (17.9%), upper respiratory tract infection (8.8%), headache (6.8%), and conjunctivitis (6.2%). Pre-defined TEAEs of special interest (eye disorders, skin infections requiring systemic treatment, eczema herpeticum, malignancies) were observed at rates similar to, or lower than, the initial treatment period of the parent trials. Serious TEAEs were reported in 9.0% of patients (EAIR: 3.54 per 100 PY), and TEAEs that led to permanent discontinuation of treatment occurred in 4.5% of patients (EAIR: 1.71).14
At Week 248, response rates (95% CIs) for IGA 0/1 and EASI-75 were 66.7% (56.1–75.8) and 92.9% (85.3–96.7), respectively (Figure 1). Improvements in itch, sleep, and QoL were sustained at levels equivalent to no-to-mild disease throughout ECZTEND.14
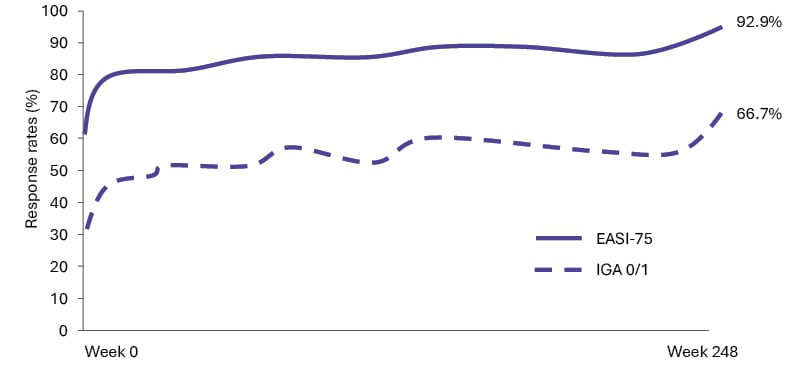
Figure 1: Proportion of patients achieving IGA 0/1 and EASI-75 as observed over 248 weeks of tralokinumab
treatment in ECZTEND.14
Graph not drawn to scale.
EASI: Eczema Area and Severity Index; EASI-75: improvement of ≥75% in EASI; IGA 0/1: Investigator’s Global
Assessment score of 0 or 1.
Blauvelt et al.14 concluded that long-term use of tralokinumab for up to 1 year in parent trials plus up to 5 years in ECZTEND was well-tolerated with no new safety signals identified, and that tralokinumab demonstrated robust long-term efficacy with sustained improvements in AD signs, symptoms, and QoL.
Efficacy of Tralokinumab in High-Burden, Difficult-to-Treat Body Areas
Head and Neck Region
Involvement of the H&N is reported in 72% of patients with moderate-to-severe AD in real-world populations.3 AD of the H&N is especially challenging to treat compared to that of other body regions; a real-world study observed that 68% of patients still displayed signs of AD in the H&N area after 2 years of systemic biologic treatment with dupilumab.15 The high visibility of this area may explain why patients with H&N AD greatly value treatments that provide complete/almost complete skin clearance.16 Recent real-world studies demonstrated the effectiveness of tralokinumab for H&N AD,17,18 and the two posters summarised below provide additional supportive findings from a post-hoc analysis of clinical trial data and an interim analysis of the real-world TRACE study.19,20
Clinical Trial Data
Ribero et al.19 presented a post-hoc analysis of data from adults with moderate-to-severe AD continuously treated with tralokinumab for up to 4 years (up to 52 weeks in ECZTRA 1 or ECZTRA 2 plus up to 152 weeks in ECZTEND). This post-hoc analysis was designed to evaluate the impact of long-term tralokinumab treatment on H&N AD and the association between improvements in H&N AD and QoL.19
By Week 152 of ECZTEND, among patients with a baseline H&N EASI score of ≥1, the majority of patients (86.1%) achieved H&N EASI ≤1, and 80.6% achieved ≥75% improvement in H&N EASI. In a subgroup of patients with baseline H&N EASI score of ≥4, most patients (75.6%) achieved H&N EASI ≤1 and 81.7% achieved ≥75% improvement in EASI (Figure 2). Improvements were comparable across body region median EASI sub-scores. Improvements in H&N EASI at Week 16 were correlated with total Dermatology Life Quality Index (DLQI) improvements, with the strongest numerical correlations observed for DLQI questions regarding skin discomfort and embarrassment due to skin.19
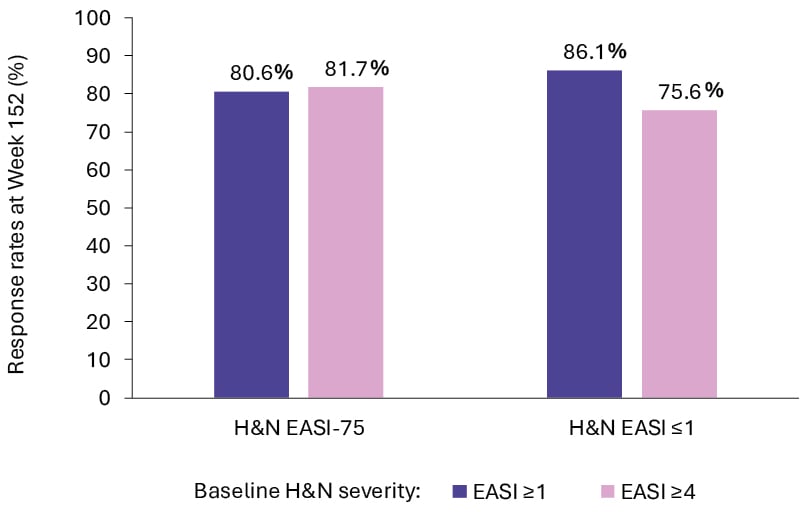
Figure 2: Improvements from baseline in atopic dermatitis severity after 152 weeks of tralokinumab treatment.19
EASI: Eczema Area and Severity Index; EASI-75: improvement of ≥75% in EASI; H&N: head and neck.
Ribero et al.19 concluded that tralokinumab treatment for up to 4 years improved H&N EASI regardless of baseline H&N AD severity, and that this correlated with improvements in QoL.
Real-World Data
Armstrong et al.20 presented an interim analysis of the non-interventional TRACE study, evaluating the effectiveness of up to 9 months of tralokinumab treatment on AD signs and symptoms in patients with H&N AD.
TRACE is an international, prospective, single-cohort study of adult patients with AD who were prescribed tralokinumab according to national approved labels. At baseline, 79.5% of patients in the full analysis set (FAS) had H&N AD (n=655/824); mean age was 42.1 years, mean AD duration was 20.6 years, 53.0% of patients were male, and 76.6% were White. At data cut-off (15th October 2023), not all patients had completed all visits.20
Among patients with H&N AD at baseline, 67.2% (363/540) still reported AD in the H&N region at 3 months. At 9 months, only 52.1% (62/119) still reported AD in the H&N region. The percentage of patients with baseline H&N AD who reported clear or almost clear skin (IGA 0/1) increased from 1.4% (9/650) at baseline to 33.6% (172/512) at 3 months, 48.4% (121/250) at 6 months, and 57.4% (58/101) at 9 months of tralokinumab treatment. Moreover, the percentages of patients who reported severe AD (IGA=4) decreased from 37.7% (245/650) at baseline to 4.7% (24/512) at 3 months, 2.8% (7/250) at 6 months, and 2.0% (2/101) at 9 months. Among patients with H&N AD and IGA ≥2 at baseline, 46.4% (220/474) achieved ≥2-point improvement in IGA at 3 months, 59.1% (140/237) at 6 months, and 71.6% (68/95) at 9 months.20
Among patients with H&N AD and DLQI ≥6 (at least a moderate effect on QoL) at baseline, the majority achieved ≥6-point reduction in DLQI with tralokinumab: 57.9% (84/145) at 3 months, 63.6% (49/77) at 6 months, and 74.4% (32/43) at 9 months. In terms of itch, mean Peak Pruritus Numeric Rating Scale (NRS) score improved from 6.4 (n=387) at baseline to 4.2 (n=213) at 3 months, 3.5 (n=111) at 6 months, and 3.3 (n=59) at 9 months of tralokinumab treatment. Similarly, patients’ quality of sleep, measured using mean Sleep NRS, improved from 5.2 (n=305) at baseline to 2.8 (n=170) at 3 months, and 2.3 at 6 and 9 months (n=84 and n=53, respectively).20
Overall, similar improvements were observed across all endpoints in both dupilumab-naïve (n=154) and dupilumab-experienced (n=501) patients with H&N AD at baseline, with higher baseline disease severity in dupilumab-naïve patients.20
Armstrong et al.20 concluded that up to 9 months of tralokinumab treatment in a real-world setting reduced AD in the H&N region and disease severity and improved QoL in patients with AD.
Genital Region
The presentation of AD on genitals may be overlooked and/or underreported due to a reluctance to discuss this sensitive area with clinicians and lack of routine examination of this region.21,22 However, among a cross-sectional, multi-country cohort of 1,474 patients, 16.6% were identified as having genital AD.4 These patients experience a significantly higher disease burden than patients without genital AD,4 and AD in this region can have a significant negative impact on patients’ QoL,4,21 including pain, sleep, mood, sexual function, and personal relationships.23,24
Recent case series demonstrated the successful use of biological therapy for AD on the genitals,21,22 and data were presented from an interim analysis of the TRACE study at FCDC 2024 that focused on the real-world effectiveness of tralokinumab in adults with genital AD.24
The analysis by Serra-Baldrich et al.25 evaluated changes in disease severity and patient-reported outcomes (PRO) among patients with genital AD with up to 3 months of tralokinumab treatment (data cutoff 15th October 2023). At baseline, 14.9% of patients in the FAS of TRACE had AD on the genitals (123/824); these patients had a mean age of 42.2 years, mean AD duration was 19.2 years, 63.4% were male, and 81.3% were White.25
Among patients with AD on genitals at baseline, only 25% of patients reported AD on genitals at 3 months (25/100). The proportion of patients reporting clear or almost clear skin (IGA 0/1) increased from 0.0% at baseline (0/122) to 31.9% at 3 months (30/94). At the same time, the proportion of patients reporting severe AD (IGA=4) decreased from 49.2% at baseline (60/122) to 7.4% at 3 months (7/94). Among patients with genital AD and IGA ≥2 at baseline, 48.9% achieved IGA ≥2-point improvement at 3 months (44/90).25
Of those patients with genital AD and DLQI ≥6 (at least a moderate effect on QoL) at baseline, DLQI ≥6-point improvement was achieved in 63.6% of patients at 3 months (14/22). In terms of sleep quality, mean Sleep NRS decreased from 6.2 at baseline (n=50) to 3.5 at 3 months (n=26).25
Serra-Baldrich et al.25 concluded that these data indicate effectiveness and improvements in patient-reported outcomes with tralokinumab in adults with AD on genitals in a real-world setting.
Comparison with Other Biologics
In addition to tralokinumab, biologic therapies approved for use in moderate-to-severe AD include dupilumab and lebrikizumab.26-29 In the absence of head-to-head trial data, indirect comparison methods that adjust for cross-trial differences, such as MAIC, can be used to compare efficacy between therapies.30 Tralokinumab and dupilumab were indirectly compared in an unanchored MAIC study which demonstrated that, in combination with TCS, these therapies had similar efficacy at 32 weeks.31
At FCDC 2024, Augustin et al.32 presented data from an anchored MAIC study comparing tralokinumab and lebrikizumab at Week 52 in Week 16 responders. Both tralokinumab and lebrikizumab are monoclonal antibodies specifically targeting IL-13, that have demonstrated efficacy in patients with moderate-to-severe AD up to 52 weeks of treatment.10,33,34
In this MAIC study, individual patient data from the ECZTRA 1/2 tralokinumab trials10,35 were compared with aggregate data from the ADvocate 1/2 lebrikizumab trials.33,34 Week 16 responders were defined as those patients achieving a 75% reduction in the EASI (EASI-75) or IGA of 0 or 1 with ≥2-point improvement (IGA 0/1) without use of topical or systemic rescue medication. In both ECZTRA 1/2 and ADvocate 1/2, Week 16 responders were re-randomised to receive placebo or active treatment (tralokinumab 300 mg or lebrikizumab 250 mg, respectively) every 2 weeks (Q2W), or every 4 weeks (Q4W), for the 36-week maintenance period. Patients who received rescue medication, discontinued treatment, or transferred to the escape arm during the maintenance period were imputed as non-response for binary endpoints and imputed as last observation carried forward for continuous endpoints. Patients in the ECZTRA trials were weighted to match those of the ADvocate trials based on baseline age, sex, ethnicity, BMI, mean AD duration, proportion with IGA=3, mean EASI, mean worst daily pruritus NRS, and Week 16 characteristics (mean EASI, proportion of IGA 0/1 response, and mean pruritus NRS).32
The study showed that tralokinumab had comparable efficacy to lebrikizumab across all endpoints. Results for the Q2W endpoints were numerically in favour of tralokinumab, though differences were not statistically significant. Response differences for outcomes for the Q2W and Q4W arms are shown in Figure 3.32
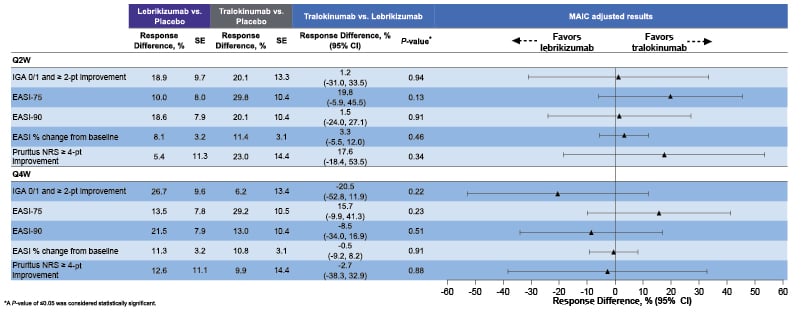
Figure 3: Response difference for achieving efficacy endpoints for tralokinumab versus lebrikizumab at Week 52.32
EASI: Eczema Area and Severity Index; EASI-75: improvement of ≥75% in EASI; EASI-90: improvement of ≥90% in EASI; IGA 0/1: Investigator Global Assessment score of 0 or 1; MAIC: Matching-Adjusted Indirect Comparison; NRS: Numerical Rating Scale; SE: standard error; Q2W: every 2 weeks; Q4W: every 4 weeks.
Augustin et al.32 concluded that, within the limitations of MAIC, comparable maintenance of efficacy was observed between tralokinumab and lebrikizumab after 52 weeks of treatment in 16-Week responders.
Efficacy data from clinical trials can be difficult to compare directly, as trial designs can differ. For example, compared to the ECZTRA trials, the ADvocate trials included patients with a shorter baseline disease duration; a second loading dose of the study drug was administered at Week 2; and the washout period for TCS, calcineurin inhibitors, and topical phosphodiesterase-4 inhibitors was 1 week rather than 2 weeks.10,33 Therefore, although the ADvocate trial outcomes appear to indicate that lebrikizumab has a greater efficacy compared to tralokinumab in the ECZTRA trials, this may be due to differences in trial design.34,35 Anchored MAIC studies can thus be helpful to compare data from clinical trials as they match patient characteristics to align study populations more closely.
Conclusion
Data presented at the EADV, FCDC, and ISAD 2024 meetings demonstrated that there were no new safety signals associated with long-term use of tralokinumab in patients aged ≥12 years with moderate-to-severe AD.14 Tralokinumab provided stable disease control, including in body areas considered most burdensome and difficult to treat, such as the H&N and genitals.19,20,25 In addition, MAIC studies showed no significant difference between tralokinumab and lebrikizumab in terms of efficacy in mild-to-moderate AD.32
Over 70% of patients with AD have H&N involvement, and this represents the most visible body region.3,15,16 As such, H&N AD deserves special consideration of the best treatment choices for this important area. In these 2024 data, it was also highlighted that genital AD may be overlooked and/or underreported despite being present in an estimated 16.6% of patients and being associated with a high disease burden and significant negative impact on the QoL.4,21,22 To successfully treat all body regions with AD, increased awareness of AD involvement in underreported sites is needed.25 Appropriate available treatment options should be used, taking into account patient preference for clearing AD from the most burdensome areas.25
| Adverse events should be reported. Reporting forms and information can be found at: www.mhra.gov.uk/yellowcard or search for MHRA Yellow card in the Google Play or Apple App Store. Adverse events should also be reported to Drug Safety at LEO Pharma by calling +44 (0)1844 347333 or e-mail: [email protected] |