BACKGROUND AND AIMS
Hepatocyte senescence has emerged as a key pathogenic driver in the onset and progression of metabolic dysfunction-associated steatotic liver disease and its advanced stage, metabolic dysfunction-associated steatohepatitis.1 In metabolic dysfunction-associated steatotic liver disease, hepatocytes undergo senescence, losing their proliferative capacity and acquiring a senescence-associated secretory phenotype (SASP). SASP factors, including pro-inflammatory cytokines, chemokines, and pro-fibrotic mediators, profoundly reshape the hepatic microenvironment, promoting lipid accumulation and chronic inflammation, and activating fibrogenic pathways, thereby accelerating the transition from simple steatosis to metabolic dysfunction-associated steatohepatitis.1-3 Recent evidence has expanded this concept, linking hyperinsulinaemia to senescence-driven metabolic dysfunction. Chronic hyperinsulinaemia directly induces cellular senescence (CS), which in turn amplifies insulin resistance (IR) and tissue dysfunction through SASP secretion, creating a self-reinforcing pathological loop.4,5 Interventions that lower insulin levels have been shown to attenuate IR, reduce hepatic steatosis, suppress inflammation, and extend lifespan. The causal role of insulin in driving CS is further supported by preclinical studies in which senolytic treatments alleviate metabolic dysfunction. Together, these results highlight a vicious cycle in which CS and hyperinsulinaemia perpetuate each other, underscoring the therapeutic value of strategies that disrupt this loop.5-7 Among these, glucagon-like peptide-1 receptor agonists (GLP-1RA) stand out as particularly promising. Beyond their glucose-lowering and weight-reducing actions, increasing evidence indicates that GLP-1RAs exert direct anti-inflammatory and anti-senescence effects. Preclinical studies show that the GLP-1RA exendin-4 reverses age-related alterations in different tissues, including liver and adipose tissue, while clinical data show that long-term GLP-1RA administration reduces systemic inflammation and mitigates metabolic decline. These observations raise the possibility that GLP-1RAs act as senotherapeutic agents.8-10
RESULTS
In this context, the authors investigated the ability of glucagon-like peptide-1 (GLP-1) and exendin-4 to counteract hepatocyte senescence induced by chronic hyperinsulinaemia. Human hepatocytes exposed to prolonged hyperinsulinaemia developed a senescent phenotype, characterised by upregulation of canonical senescence markers (ZMAT3, p53, p21, phosphorylated γH2AX) and SASP factors (CXCL8, IL-18, and MMP3). Treatment with GLP-1 or exendin-4 markedly attenuated the expression of both senescence markers and SASP mediators, demonstrating that GLP-1 and exendin-4 can suppress the senescence programme triggered by hyperinsulinaemia. Because GLP-1 metabolism is regulated by dipeptidyl peptidase-4 (DPP-4), the authors next examined whether modulating DPP-4 impacts hepatocyte senescence. Silencing DPP-4 alone reduced senescence markers and SASP mediators. Furthermore, combining DPP-4 silencing with GLP-1 or exendin-4 treatment produced an additive reduction in senescence and SASP factors, suggesting that dual targeting of DPP-4 and GLP-1 signalling may provide synergistic protection against hepatocyte senescence.
CONCLUSION
Collectively, the authors’ findings provide evidence that GLP-1 and exendin-4 protect hepatocytes from senescence, and that lowering DPP-4 levels enhances this protective effect (Figure 1). These results uncover a previously unrecognised mechanism of GLP-1RAs and support their broader potential in reducing systemic metabolic dysfunction. Importantly, combining GLP-1RAs with lifestyle interventions and other senescence-targeted strategies could break the vicious cycle linking IR and CS, thereby slowing disease progression and enhancing metabolic health.
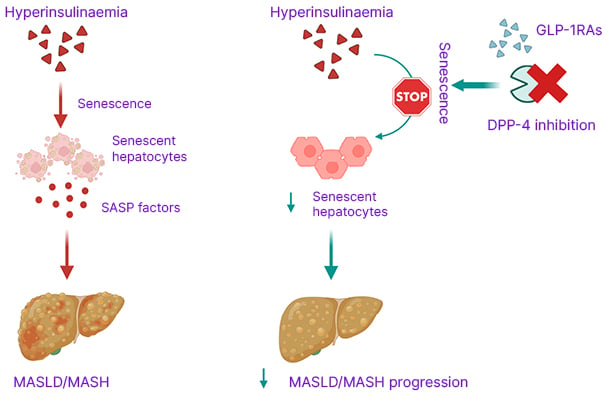
Figure 1: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 targeting protect against hepatocyte senescence.
Created using BioRender.com.
DPP-4: dipeptidyl peptidase-4; GLP-1RAs: glucagon-like peptide-1 receptor agonists; MASH: metabolic dysfunction- associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; SASP: senescence- associated secretory phenotype.