Abstract
Sepsis remains a significant cause of maternal mortality, and is responsible for approximately 11% of maternal deaths worldwide. Group A β-haemolytic Streptococcus (GAS) is one of the pathogens linked to maternal sepsis. It is notable for its virulence, rapidly progressing to invasive group A streptococcal (iGAS) infections with a high mortality rate of 20–40%. The authors report a case of puerperal sepsis due to GAS in a postpartum woman who initially presented with limb swelling following an uneventful vaginal delivery. Within hours, she rapidly deteriorated into septic shock, despite early initiation of the Sepsis Six pathway. GAS was isolated from both the genital tract and blood, even though classic symptoms of endometritis were absent. Intensive treatment, including broad-spectrum antibiotics, fluid resuscitation, intravenous Ig, intensive care support, and surgical debridement successfully saved her life. However, she experienced prolonged morbidity due to extensive tissue loss from wound debridement. Although iGAS is rare in the UK, pregnant and postpartum women have a significantly increased risk, particularly within the first 28 days postpartum. Puerperal GAS infections often present atypically, with non-specific symptoms rather than classical signs of endometritis. Given the high mortality associated with iGAS, a high index of clinical suspicion, early recognition, and aggressive management are critical to improve the outcome.
Key Points
1. Pregnant women have a 20-fold higher risk of invasive Group A streptococcal infections than non-pregnant women, rising to 80-fold in the first 28 days postpartum.2. Puerperal invasive Group A streptococcal infections may present with atypical symptoms and progress rapidly to life-threatening conditions such as necrotising fasciitis, streptococcal toxic shock syndrome, disseminated intravascular coagulation, and septic shock.
3. A high index of suspicion, prompt administration of broad-spectrum antibiotics, fluid resuscitation, urgent escalation, multidisciplinary management, and early identification and treatment of the source of infection (including surgical management if appropriate) are crucial in reducing morbidity and mortality.
INTRODUCTION
Sepsis continues to be a significant contributor to maternal mortality, and is responsible for approximately 11% of maternal deaths worldwide.1 According to the MBRRACE 2024 report, sepsis accounted for 25/275 maternal deaths during the 2020–2022 triennium, representing 9% of cases, with a maternal moratality rate of 0.89 per 100,000 maternities due to pregnancy-related infection.2 Among the pathogens linked to maternal sepsis, Escherichia coli and group A β-haemolytic streptococci (GAS) are notable for their association with severe morbidity and mortality.3 GAS, in particular, is recognised for its heightened virulence, with rapid progression to life-threatening invasive group A streptococcal (iGAS) infections, such as endometritis, necrotising fasciitis (NF), streptococcal toxic shock syndrome (STSS), septic shock, multiorgan failure, and death.3,4 Although iGAS is relatively uncommon in the UK, with an annual incidence of two to four cases per 100,000 individuals, pregnant women face a 20-fold greater risk of developing iGAS infections compared to non-pregnant women, and this risk increases up to 80-fold within the first 28 days postpartum.5 Furthermore, pregnancy-related iGAS infections carry a high mortality rate of 20% within 7 days of diagnosis, rising to over 40% if septic shock occurs.6 Even though the vaginal colonisation of GAS is low (ranging 0.03–0.37%), ascending infections can lead to severe invasive infections, increasing morbidity and mortality.7
Although endometritis typically presents with symptoms such as abdominal pain, fever, abnormal vaginal discharge, or bleeding, puerperal iGAS infections often manifest with non-specific symptoms such as malaise, fatigue, or other atypical features.6 These may include severe limb pain or swelling, blisters, infected episiotomy wounds, NF involving the uterus and cervix, and shock. While NF following vaginal delivery is rare, it has been documented.3 Tissue invasion at the site of vaginal injury can lead to the septicaemic spread of GAS to the skin and soft tissue, resulting in NF. A hallmark of NF is severe pain requiring escalating doses of analgesics such as opioids. In its early stages, NF may present with minimal skin changes, as the infection begins in deep tissues. As the condition progresses, it may cause blisters and necrosis.6 The absence of fever and the presence of watery, inoffensive lochia in cases of GAS can mislead clinicians, potentially delaying diagnosis. Therefore, maintaining a high index of clinical suspicion is critical for early detection and timely intervention, which are essential to reducing morbidity and preventing fatal outcomes.3
CASE REPORT
A 32-year-old woman presented to obstetric triage 6 days after an uneventful, midwifery-led, vaginal birth and episiotomy with sudden onset of swelling and pain in the upper and lower limb on the same side, accompanied by a general sense of feeling unwell for the past 24 hours. At the time of presentation, her main concern was swelling rather than pain, and she had no history of injury or skin discolouration. The woman had occasional chills but recorded a normal temperature at home. There were no typical symptoms of endometritis or genital tract infection, such as abdominal pain, fever, abnormal lochia, or vaginal bleeding. Her past medical history was unremarkable. She had a history of cannabis use but denied any intravenous drug use. Her labour and birth were uneventful, with no epidural or prolonged rupture of membranes, and both mother and baby had been discharged on the day of birth. The woman confirmed that she had no similar prior symptoms, and had been well until 24 hours before the issues arose. Her baby and other family members were in good health.
Upon admission, the woman appeared unwell but haemodynamically stable, with a pulse rate of 86 bpm, blood pressure of 112/71 mmHg, respiratory rate of 18 bpm, and SpO2 of 99%, though she had a mild fever of 37.7 °C. Her Modified Early Obstetric Warning Score (MEOWS) was 0, and these parameters were comparable to the levels seen in labour. Her upper limb was more swollen and tender than her lower limb. Distal pulses were palpable. Other examinations, including cardiovascular, respiratory, and neurological systems, were unremarkable, with minimal movement limitations due to pain and swelling. Abdominal and pelvic examination revealed a 14-week involuting, nontender uterus; a clean, slightly dehisced episiotomy; and healthy lochia. A high vaginal swab was sent for culture to rule out genital tract infection on high suspicion. Differential diagnoses at this stage included deep vein thrombosis (DVT), thrombophlebitis, and early cellulitis of the upper limb with possible sepsis.
The full blood count with differential showed leukocytosis with elevated white blood cells (15.6×109/L), elevated neutrophils (14.2×109/L), and elevated C-reactive protein (418 mg/L), with normal haemoglobin (113 g/L) and platelet count (238×109/L). Electrolytes and liver function tests were deranged, with hyponatraemia (131 mmol/L), hypokalaemia (3.2 mmol/L), high bilirubin (36 µmol/L), high alkaline phosphatase (449 U/L), and high alanine transaminase (69 U/L). Renal function tests and coagulation profile were normal. The venous blood gas showed raised lactate (2.6 mmol/L) and a normal pH and glucose.
As inflammatory markers were high, sepsis management was initiated with broad-spectrum antibiotics and fluid resuscitation following blood culture collection, although the source of infection was unclear. A throat swab was taken to identify other potential infection sources. A CT scan of the abdomen and pelvis was performed as ultrasound was not available out of hours, which suggested the possibility of endometritis, though the uterine cavity appeared empty and there were no clinical signs suggesting endometritis. The patient was referred to the medical team for a Doppler ultrasound of her limbs to rule out DVT, and was started on anticoagulation therapy in the interim.
Within 3–4 hours, she developed a persistent tachycardia (pulse rate: 130 bpm), tachypnoea (respiratory rate: 35 beats per minute), and intermittent altered mental status, with a MEOWS of 6. Biochemical markers began to deteriorate further, with abnormal liver and renal function tests, metabolic acidosis (pH: 7.2), lactate (5.6 mmol/L), and low bicarbonate (15 mmol/L; Table 1). A senior escalation and multidisciplinary team meeting involving the outreach, emergency, and intensive therapy unit (ITU) teams was conducted, and the woman was transferred to ITU. Antibiotic therapy was escalated to empirical meropenem and gentamicin, according to the microbiologist’s recommendation. Despite aggressive fluid resuscitation and sepsis management, the woman deteriorated into shock.
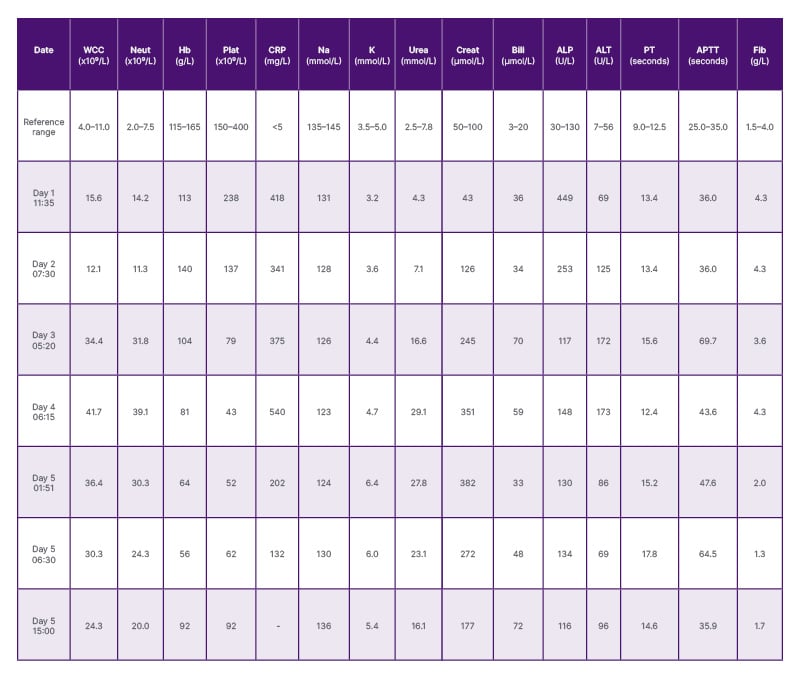
Table 1: Table showing blood results of the patient from Day 1 to Day 5 of sepsis.
ALP: alkaline phosphatase; ALT: alanine transaminase; APTT: activated partial thromboplastin time; Bili: bilirubin;
Creat: creatinine; CRP: C-reactive protein; Fib: fibrinogen; Hb: haemoglobin; K: potassium; Na: sodium;
Neut: neutrophil count; plat: platelets; PT: prothrombin time; WCC: white cell count.
Notably, apart from a swollen limb, there were no other localised signs of infection. Differential diagnoses such as acute fatty liver of pregnancy and peripartum cardiomyopathy were considered due to the unusual presentation and rapid deterioration, although the evidence for these was not conclusive. CT head and Doppler ultrasound of the limbs ruled out intracranial pathology and venous thromboembolism, respectively. Over the following 10–12 hours, the limb swelling worsened, and skin mottling appeared on the chest and abdomen, raising concerns for toxic shock syndrome. By Day 2, the patient had developed a persistent high fever, skin blisters, and mild discolouration suggestive of early NF. Blood and genital cultures confirmed iGAS infection (type emm77). Antibiotics were adjusted to meropenem and clindamycin, with the latter chosen to mitigate exotoxin production based on microbiological input. Orthopaedics, general surgery, and plastic surgery teams were consulted, and infection control measures were implemented, notifying the infection prevention and neonatology teams.
The woman exhibited classical features of STSS with NF, multiorgan dysfunction, disseminated intravascular coagulation, and stress cardiomyopathy. She required intubation, inotropic support, intravenous Ig (IVIG) therapy, intravenous albumin, and blood products. Imaging (CT and MRI of the limbs; Figure 1) revealed extensive muscle oedema and myositis, though a CT limb angiogram ruled out arterial thrombosis. Repeat transvaginal ultrasound and MRI pelvis suggested a possible small intrauterine clot or retained products of conception (RPOC).
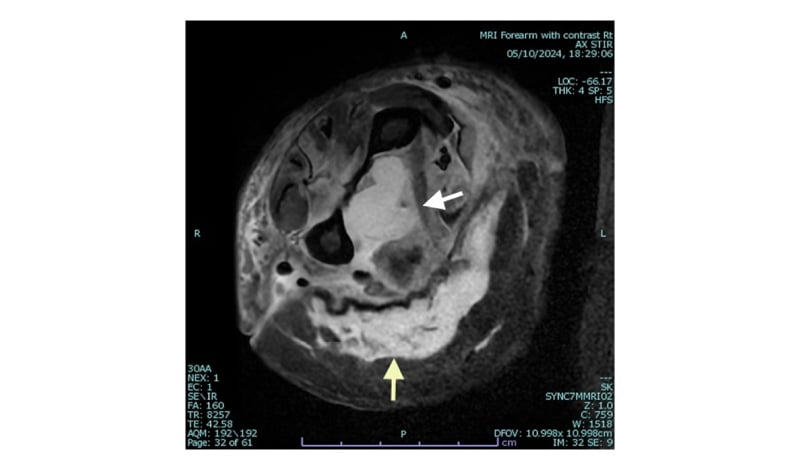
Figure 1: MRI image of the mid forearm with subcutaneous oedema.
Axial STIR sequence MRI image at the level of the right mid forearm showing extensive high signal change abnormality in the flexure compartment musculature (white arrow) with surrounding subcutaneous oedema (yellow arrow).
STIR: short tau inversion recovery.
On Day 4, after stabilising the patient, surgical intervention was undertaken in her best interest as she was still intubated, which included fasciotomy, extensive debridement of the upper and lower limbs, lower abdominal wall, and uterine evacuation to address potential RPOC. Interestingly, no RPOC was identified. GAS was isolated from both deep tissue and muscle samples. Clindamycin resistance was detected on extended culture, prompting its replacement with linezolid alongside meropenem. Amputation was considered as part of her ongoing management after stabilising her coagulation.
The woman showed gradual improvement with multiple extensive debridements, meticulous wound care, and ITU support, alongside intravenous antibiotics, IVIG, albumin, and blood products. By Day 6, blood and genital swab cultures were sterile. On Day 10, she was extubated and transferred to a tertiary unit for plastic surgery for skin grafting and reconstructive procedures, which required multiple repeat surgeries for wound revision and reconstruction.
Prolonged antibiotic therapy led to complications, including Clostridium difficile diarrhoea. After 2 months of hospitalisation, the patient was discharged with a comprehensive plan for ongoing antibiotic therapy, neuropathic pain management, physiotherapy, occupational therapy, and mental health support for both her and her family. Despite extensive tissue loss, amputation was fortunately avoided.
DISCUSSION
Group A Streptococcus is a facultative, gram-positive coccus categorised under β-haemolytic streptococci.8 It is highly contagious and spreads through close contact via respiratory droplets, direct skin contact, or indirectly through fomites such as towels or bedding. GAS can colonise the throat, skin, and occasionally the vagina and anogenital tract, and is usually asymptomatic.4 GAS is associated with a spectrum of clinical infections, ranging from mild, non-invasive conditions like pharyngitis, scarlet fever, and impetigo, to severe, life-threatening invasive infections.8 Approximately 90% of iGAS cases are community-acquired, with the remainder being nosocomial, including reported outbreaks in healthcare institutes.7
iGAS is identified by detecting GAS through culture or molecular methods, such as PCR, from normally sterile body sites, including blood, body fluids, bone, endometrium, or deep tissues.5 Severe infections resulting from iGAS include bacteraemia, STSS, puerperal sepsis, and NF. While specific risk factors for iGAS are often not evident, recent respiratory infections, particularly influenza, have been recognised as significant predisposing factors.9
Pregnancy-related iGAS infections are most frequently reported in late pregnancy (beyond 30 weeks’ gestation) and up to 4 weeks postpartum.9 This heightened risk is attributed to immunological changes during pregnancy and the potential GAS invasion through perineal trauma or ascending infection via the endocervix.9
The M protein is a key virulence factor of GAS, enabling the bacterium to invade sterile sites, evade phagocytosis, and adhere to epithelial cells.9 GAS also produces exotoxin A, a superantigen that triggers an intense inflammatory response characterised by excessive activation of circulating T cells and cytokines, resulting in capillary leak syndrome and severe hypotension.9 The M protein gene (emm) encodes the surface M protein.9 Currently, emm typing is the molecular gold standard for GAS typing globally.3 emm3 is the most common type among more than 200 described emm types in the 2024–2025 season in the UK.10 Certain emm types are associated with specific clinical conditions; for example, emm28 is linked to puerperal sepsis, although GAS, being a versatile pathogen, is often linked with an array of presentations with varying severity.7 emm77 was the strain detected in this patient.
Typically, symptoms arise within 2–4 days post-delivery and include fever, chills, and tachycardia in endometritis. However, non-specific symptoms or atypical manifestations are not uncommon. Signs of infection, such as pyrexia, may not always be present and may not correlate with the severity of sepsis. In contrast, hypothermia should raise an early suspicion of iGAS. Lochia may appear serosanguinous or watery due to GAS-induced leukocyte destruction, and may not have an offensive odour.11 Absence of classic features of endometritis, such as fever, abdominal and pelvic pain, purulent lochia, and uterine tenderness can lead to diagnostic delay, increasing the risk of unexpected sudden deterioration to life-threatening complications despite aggressive sepsis management.3
For life-threatening sepsis, a combination of piperacillin and tazobactam or meropenem and clindamycin provides extended broad-spectrum cover.3 If methicillin-resistant Staphylococcus aureus is suspected, vancomycin should be added.
Early identification and source control through surgical intervention, such as evacuation of RPOC, abscess drainage, fasciotomy, wound debridement, or hysterectomy, are critical in preventing rapid disease progression. However, identifying the source of infection is not always possible. Therefore, a high index of suspicion, adherence to the ‘Think Sepsis’ programme, and early initiation of sepsis pathway protocol, when in doubt, is essential.2 This includes early sepsis risk assessment, initial fluid resuscitation, prompt administration of broad-spectrum intravenous antibiotics after blood culture within 1 hour of presentation, escalation to senior medical staff, multidisciplinary input, intensive care support, and source control.3
In this patient, the primary complaint of limb swelling and pain initially raised suspicion of DVT as the leading differential diagnosis. However, persistent tachycardia and elevated inflammatory markers pointed towards possible sepsis, despite the atypical presentation. The absence of pelvic pain and normal temperature, as well as healthy lochia, complicated the diagnosis of endometritis, even though CT abdomen suggested its possibility. Although broad-spectrum antibiotics and fluid resuscitation were initiated promptly, the patient’s rapid progression to shock with multiorgan dysfunction prompted consideration of alternative diagnoses, such as acute fatty liver of pregnancy or peripartum cardiomyopathy. Ultimately, the isolation of group A Streptococcus from vaginal samples and evidence of bacteraemia confirmed the diagnosis of iGAS infection.
As the patient developed symptoms on the 6th daypostpartum, it is likely that the infection was community-acquired, as hospital-acquired infections typically manifest within 2–4 days of discharge. On reflection, the progression of NF followed a characteristic course, starting with limb swelling and pain and advancing to blister formation and eventual tissue necrosis.
IVIG plays a critical role in severe, unresponsive cases of iGAS infections, such as NF and STSS, by neutralising exotoxins and superantigens.3 It is recommended only for patients who are critically ill, and doses of up to 2 g/kg have been safely used in exotoxin-mediated sepsis during pregnancy without adverse effects.3 The combination of intravenous Ig with clindamycin is synergistic and highly effective in managing suspected GAS sepsis during pregnancy, offering enhanced therapeutic benefits.3Hysterectomy may be required in cases of iGAS infections involving uterine or adnexal necrosis.12 However, in the authors’ patient, this was fortunately avoided, and only ultrasound-guided suction evacuations were needed for suspected RPOC. Despite this, the morbidity associated with repeated extensive wound debridement was significant and debilitating, with extremely limited function of the affected limbs in addition to neuropathic pain. Survivors of iGAS infections often endure severe long-term complications, including prolonged hospitalisations and the need for multiple surgical interventions, such as amputations or extensive wound care. In this case, the patient required ongoing physiotherapy, occupational therapy, and comprehensive management of neuropathic pain, along with mental health support, after discharge. Given the traumatic nature of the experience, emotional and psychological support should also be extended to family members to help them cope and recover alongside the patient.
Raising awareness among the pregnant population about GAS infection, its signs and symptoms, and the importance of personal hygiene is crucial.7 This includes emphasising practices such as washing hands thoroughly before and after using the lavatory to prevent perineal contamination.3
Puerperal iGAS infection is a rare condition; however, a recent resurgence has been observed, attributed to increasing bacterial virulence. It is associated with extremely high morbidity and mortality rates. Even in cases of survival, patients often endure significant debilitating complications, requiring a prolonged recovery period. Successful outcomes depend on maintaining high clinical vigilance, ensuring prompt diagnosis, timely escalation of care, multidisciplinary involvement, early initiation of antibiotics, fluid resuscitation, and effective source control of the infection. Additionally, comprehensive support for the patient and their family should be provided even after hospital discharge to aid in their return to normality.