Abstract
Actinomycosis is an uncommon, chronic, and slowly developing granulomatous infection observed in individuals who are immunocompromised and those who are immunocompetent. Thoracic actinomycosis is particularly challenging to diagnose due to its varying presentations, which can mimic bronchogenic carcinoma, pneumonitis, or tuberculosis-like infections. In some cases, bronchopleural fistulas have been reported. The authors report a case of a 38-year-old male presenting with dysphagia and weight loss. A series of radiological assessments were conducted. The chest X-ray revealed fine reticular opacities, while a high-resolution CT of the thorax showed centrilobular nodules affecting both lungs. A contrast-enhanced CT of the thorax identified a mass in the lower oesophagus, as well as circumferential wall thickening in the lower third of the oesophagus and the gastro-oesophageal junction. Oral contrast imaging demonstrated a fistulous tract originating at the gastro-oesophageal junction and extending superolaterally to the right lower bronchus. Despite initial concerns for oesophageal carcinoma, a biopsy confirmed an infection caused by Actinomyces. The nonspecific clinical presentation and radiological findings can make diagnosing pulmonary actinomycosis difficult, but they remain valuable for clinicians when considering differential diagnoses.
Key Points
1. Oesophageal actinomycosis is a rare cause of dysphagia and can be mistaken for cancer. This case report underscores the importance of taking notice of underlying local factors in a patient who is otherwise immunocompetent.
2. Imaging and clinical findings of oesophageal actinomycosis closely resemble those of squamous cell carcinoma. The diagnosis of oesophageal actinomycosis involves imaging studies, endoscopic biopsy, and tissue sampling for histopathological analysis.
3. Timely and accurate diagnosis is very important, as surgery is indicated for oesophageal actinomycosis complications. Successful management depends on thorough patient education and counselling, owing to a long-term antimicrobial regimen.
INTRODUCTION
The Actinomyces family includes 47 different species. Actinomycosis infections are characterised by abscess formation, granulation, dense fibrotic tissue development, and cutaneous sinuses that discharge ‘yellow sulfur’ granules. In some cases involving the lungs, cavitations can form and may eventually lead to sinus tracts opening to the skin. Gastrointestinal infections may result in fistula formation, while bone involvement often leads to permeative bone destruction.1
The most frequently encountered form of Actinomyces infection is cervicofacial actinomycosis, which accounts for 50% of all cases. Thoracic actinomycosis represents 15–20% of cases, while abdominopelvic involvement occurs in approximately 20%. Less commonly, Actinomyces can infect the central nervous system, bones, muscles, and prosthetic joints.2
A functional immune response plays a key role in controlling actinomycosis. Factors that suppress this response, such as chemotherapy, HIV infection, organ transplants (e.g., lung or renal), or the use of steroids, can increase the risk of developing an infection. Additional risk factors include being male, aged 20–60 years, having poor oral hygiene, diabetes, alcohol use, trauma, recent surgery, or radiation exposure.3
Diagnostic imaging, including barium swallows, oral contrast CTs, and contrast-enhanced CTs, is vital for differentiating actinomycosis from conditions like oesophageal tuberculosis or neoplasms.4 On CT and PET/CT scans, pulmonary actinomycosis often appears as a slowly progressing mass, which can be mistaken for a malignancy.5,6 Clinicians should be aware of the unusual presentation of oesophageal actinomycosis and its tendency to mimic cancer before surgery.
In this report, the authors present a rare case of oesophageal actinomycosis in a patient who is immunocompetent and has experienced symptoms including chronic cough, chest pain, dysphagia, and weight loss, closely resembling a malignancy.
CASE REPORT
A 38-year-old man presented with chronic cough, chest pain, dysphagia, weight loss, odynophagia, and vomiting that had all been going on for 9 months. The patient had dysphagia to both solids and liquids (more severe with solids than liquids), and had frequent episodes of vomiting that usually occurred after meals. These symptoms were accompanied by coughing fits with sputum production and intermittent exacerbations. The patient had lost 20 kg over 1 year and had a history of chronic alcoholism and tooth extraction with poor oral hygiene.
Upon physical examination, the patient appeared with symptoms of paleness, cachexia, and hypotension. Laboratory investigations showed anaemia, leukocytosis, and a negative serology for HIV. Chest radiographs showed fine reticular opacities in the left perihilar region (Figure 1A). A high-resolution CT chest showed centrilobular nodules involving both lungs. Cavitatory subsegmental consolidation was seen in the posterobasal segment of the right lower lobe (Figure 1B).
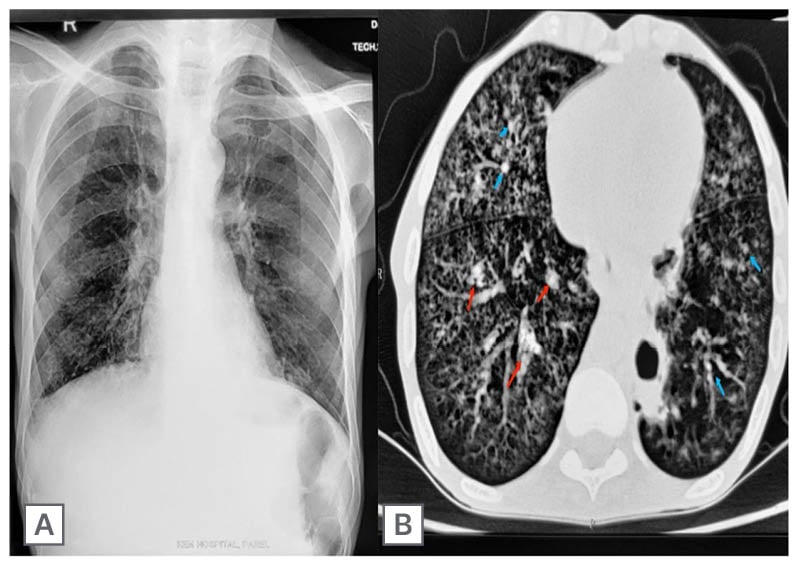
Figure 1: Chest radiograph and high-resolution CT thorax.
A) Chest radiograph showed fine reticular opacities in the left perihilar region. B) High-resolution CT thorax showed centrilobular nodules (blue arrows) involving both lungs. Cavitatory subsegmental consolidation (red arrows) is seen in the posterobasal segment of the right lower lobe.
Further imaging showed dilation of the thoracic oesophagus, with circumferential wall thickening in the lower third of the oesophagus and the gastro-oesophageal (GO) junction. There was an extension into the stomach, with a maximum wall thickness of 20 mm and a loss of distensibility. Fat planes adjacent to the liver were lost. Multiple peripherally enhancing mass lesions were seen in the subdiaphragmatic and subhepatic spaces, as well as in the left lobe of the liver (Figure 2A).
A CT scan with oral contrast showed fistulous communication originating at the GO junction and coursing superolaterally up to the right lower bronchus (Figure 2B).
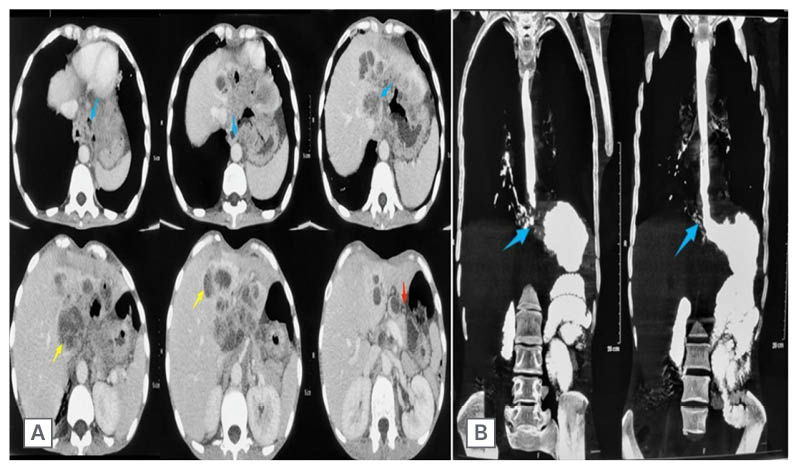
Figure 2: Contrast-enhanced CT thorax and oral contrast CT.
A) Contrast-enhanced CT thorax showed circumferential wall thickening (blue arrows) at gastro-oesophageal junction. Multiple peripherally enhancing mass lesions (yellow arrows) are seen along liver surfaces, with loss of fat plane as well as stomach distensibility (red arrows). B) Oral contrast CT showed fistulous communication at the gastro-oesophageal junction, which was coursing superolaterally up to the right lower bronchus (blue arrows).
A barium swallow confirmed the CT findings: a concentric irregular narrowing involving the retrocardiac and epiphrenic oesophagus, with proximal barium hold-up (Figure 3A). The endoscopy of the patient was attended. During the endoscopy, upon reaching the lower oesophagus, two openings were encountered: one was the GO junction, and the other was the broncho-oesophageal fistula. The oesophageal mucosa is nodular with areas of ulceration in the epiphrenic segment. Serpiginous tracts and monstrous ulceration were also observed along the lesser curvature of the stomach. At the GO junction, there were whitish exudates that could not be washed off. A fistulous communication was seen just adjacent to the GO junction (at a level of 39 cm; Figure 3B)
The differential diagnosis included tuberculosis, neoplasms, and actinomycosis. Biopsies taken from the oesophagus revealed discrete sulfur granules consistent with an Actinomyces infection, and this was confirmed upon a histopathological examination (Figure 3C). The patient subsequently received a high dose of intravenous penicillin for 4 weeks. The patient improved after this, and was changed to oral penicillin. Following medical treatment, the patient improved clinically, and at 10 weeks, a follow-up ultrasonography revealed no left lobe liver lesions. Following therapy, the patient subsequently recovered, and no additional invasive testing or imaging was required.
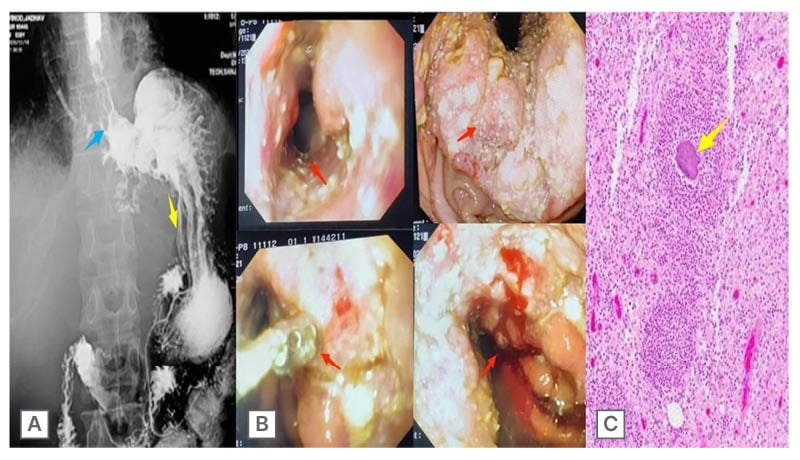
Figure 3: Barium swallow, endoscopy, and biopsy results.
A) Barium swallow confirmed the CT findings of a concentric irregular narrowing (blue arrow) involving the
retrocardiac and epiphrenic oesophagus, with proximal barium hold-up. B) Endoscopy revealed that oesophageal mucosa is nodular, with areas of ulceration in the epiphrenic segment (red arrows). Serpiginous tracts and monstrous ulceration were also observed along the lesser curvature of the stomach. At the gastro-oesophageal junction, there were whitish exudates with fistulous communication (red arrow) seen just adjacent to the gastro-oesophageal junction (at a level of 39 cm). C) Biopsies taken from the oesophagus revealed discrete sulfur granules (yellow arrows) consistent with Actinomyces infection, which was confirmed upon histopathological examination.
DISCUSSION
Actinomyces is an anaerobic, gram-positive bacillus commonly found in the normal flora of the gastrointestinal and genitourinary tracts. These organisms are slow-growing and of low virulence, often taking months to develop into infections. Therefore, they are frequently mistaken for malignancies or other diseases such as tuberculosis, candidiasis, herpes simplex virus, or cytomegalovirus.4
Actinomycosis seldom stands alone, often involving additional pathogens such as Actinobacillus, Eikenella, Enterobacteriaceae, Fusobacterium, Streptococcus, and Candida. Isolation of Actinomyces from Nocardia, using the acid-fast stain, is pivotal. In this case, only the microorganism Actinomyces, which produces sulfur granules (frequently with club-shaped ends), was present.4,7 However, sulfur granules are only present in about half of cases; hence, the absence of sulfur granules does not rule out an actinomycosis infection.7
Pulmonary actinomycosis is rarely encountered in thoracic surgery. As it were, 13 cases of pulmonary actinomycosis were distinguished among 2,247 patients with radiological pulmonary opacities, aligning with the Rizzi et al.8 study. Actinomyces israelii, a commensal organism of the oropharyngeal flora, typically causes infection following disruption to the mucosal barrier. Pulmonary involvement typically results from aspiration, particularly in individuals with poor oral hygiene, and accounts for approximately 15% of all actinomycosis cases.9 Aspiration of oropharyngeal or gastrointestinal secretions is a key mechanism of transmission to the respiratory tract.10
Fever, productive cough, dyspnoea, haemoptysis, pleuritic chest pain, weight loss, anaemia, and leukocytosis are the most common clinical symptoms of pulmonary actinomycosis.11-13 Due to these nonspecific symptoms, pulmonary actinomycosis is frequently misdiagnosed as a malignancy or another serious infection.10 Actinomycosis is found worldwide and may affect any age group. This pathogen is normally found in the oral cavity and gastrointestinal tract of healthy individuals.
In this case, the key indicators prompting the consideration of actinomycosis are poor oral hygiene, odynophagia, and weight loss.9,10 Although Actinomyces typically causes infections in the cervicofacial region, it can also affect the thorax, abdomen, and pelvis.7 Radiological findings in actinomycosis include mucosal abnormalities, such as ulcers or fistulas, on oesophagogram, while a CT scan shows oesophageal wall thickening in numerous cases.13 The gold standard for opinion is the discerning of filamentous bacteria and sulfur granules by histology or culture. However, recent antibiotic use, co-infections, or improper specimen handling may hinder culture results.13-15 Thoracic actinomycosis is particularly rare and may mimic conditions such as tuberculosis or lung cancer, both primary and metastatic.16-18 Other pathologies can also accompany actinomycosis in some cases. Lung, pleura, mediastinal, or chest wall infections may result from direct spread from a cervicofacial infection, oesophageal perforation, aspiration, or even hematogenous dissemination from a distant site.16
During radiographic examinations, pulmonary actinomycosis can appear as infiltrates, cavitary lesions, or tumour-like masses.19-22 Bone changes and pleural involvement may also be observed, though these findings are not specific to actinomycosis.22,23 The lack of distinctive clinical or imaging findings makes diagnosing actinomycosis particularly challenging. With oesophageal involvement, a barium swallow may show deep ulcers, fistulas, or sinus tracts.24,25 However, they can also reveal more common mucosal irregularities, ulcers, or strictures similar to those seen in infections like candidiasis, herpes simplex virus, or cytomegalovirus.26 CT imaging typically shows oesophageal wall thickening in the early stages of infection, potentially progressing to sinus tract formation.24
Ultimately, despite the value of radiologic findings, a definitive diagnosis requires a bacterial culture or histopathological examination of biopsy specimens, where filamentous bacteria and sulfur granules are identified.27
Based on inference, actinomycosis is a rare infection. Due to its ability to mimic other infections or malignancies, such as tuberculosis or adenocarcinoma, it is often misrecognised, particularly in young adults.4 These infections typically occur after an insult to the oesophageal mucosa, such as prior surgery or instrumentation, trauma, or infection.14 They can present as submucosal masses, ulcers, fistulas, abscesses, strictures, or draining sinus tracts.7 Thoracic actinomycosis has an excellent prognosis with early recognition and appropriate treatment, which underscores the importance of considering actinomycosis in cases that initially appear to be tuberculosis or neoplasms.28 Early diagnosis may prevent severe complications and unnecessary surgery.
CONCLUSION
Actinomyces is a slow-growing anaerobic bacterium that can cause hidden infections, leading to complications such as fistulas, abscesses, and strictures.
This case highlights the importance of distinguishing actinomycosis from malignancy, as a misdiagnosis can lead to unnecessary and invasive procedures.
Clinicians should consider actinomycosis as part of the differential diagnosis, especially when clinical findings do not align with more common conditions such as tuberculosis or neoplasms. Oesophageal actinomycosis is a rare but important cause of oesophageal pathology, particularly in patients who are immunocompromised. Radiologic findings in actinomycosis, including oesophageal wall thickening on CT and potential sinus tracts and mucosal irregularity/ulceration on a double-contrast oesophagogram, can be suggestive of the entity but are nonspecific. A biopsy with culture and histopathology should always be performed to confirm the diagnosis and avoid surgical interventions. Early recognition and an accurate diagnosis of actinomycosis can prevent serious complications and ensure effective, conservative treatment with antibiotics.