Abstract
The introduction of nivolumab has changed the landscape of relapsed/refractory (R/R) classical Hodgkin’s lymphoma (HL) treatment. Despite its clinical importance, this therapy may remain inaccessible for a significant number of patients worldwide, especially in low-income countries, due to its high cost. The recommended dose is usually 3 mg/kg (240 mg flat dose) every 15 days, which incurs a huge financial burden to patients. Currently, this therapy is used for the treatment of adult patients with R/R classical HL after autologous stem cell transplant and treatment with brentuximab vedotin.
In one study, the authors used a flat dose of 40 mg of nivolumab every 15 days in R/R classical HL, with an objective response rate of 70%, and 43.3% of patients achieved complete response. Median progression-free survival was 18.4 months with this low- and fixed-dose regimen of 40 mg.
This is a case report of a 21-year-old girl, who was previously treated with two lines of therapy and had <1-year disease-free interval after the first line of therapy. She then exhibited progressive disease, which was followed by salvage chemotherapy. She was treated with single-agent low-dose nivolumab, achieving complete response after 2 months of therapy, and was taken up for transplant. This opens up a possible option of low-dose nivolumab for patients with R/R HL as a bridge to transplant.
Key Points
1. Relapsed/primary progressive Hodgkin’s lymphoma responds well to a short course of nivolumab, permitting consolidation with stem cell transplant.2. There was an objective response rate of 70%, with 43.3% achieving complete response. This is comparable to standard-dose nivolumab trials (which report approximately 65–73% objective response rate, and approximately 40% complete response).
3. Low-dose nivolumab (40 mg every 2 weeks) maintains high efficacy with excellent tolerability and offers dramatic cost savings by three-to-seven-fold, representing a strong rationale for further trials and broader use in patients with classical Hodgkin’s lymphoma, particularly in low- and middle-income settings.
INTRODUCTION
Magnitude of the Problem
Up to a quarter of patients with classical Hodgkin’s lymphoma (HL) are resistant or have disease relapse after first-line chemotherapy.1 For such patients with relapsed or refractory (R/R) disease, second-line chemotherapy and autologous haematopoietic stem cell transplantation (ASCT) may lead to sustained remission in about half of cases.2 Historically, patients who failed ASCT had a dismal prognosis, with a life expectancy of <2 years.3-5
Existing Treatments and Limitations
Brentuximab vedotin (BV), an anti-cluster of differentiation 30 (CD30) immunoconjugate, has demonstrated activity after ASCT failure, with overall response (OR) and complete response (CR) rates of 72% and 33%, respectively. Despite high OR, most patients eventually progress or relapse, with a median progression-free survival (PFS) of 9.3 months, while up to 15% may remain in sustained CR without further medical intervention.6 Thus, even though this is a targetable option, it doesn’t offer a good, long-term sustained response, and the cost involved is around 4,500 USD per dose. This makes it unaffordable for quite a large population of patients with HL. It also results in significant Grade 3 and Grade 4 neutropenia in 33–54% of patients, with 18% of cases needing admission, and Grade 3–4 peripheral neuropathy in 10–20% cases (in the ECHELON trial).7 This leads to lots of morbidity in patients, along withfinancial toxicity.
Existing Evidence in Favour of Nivolumab
Nivolumab is a monoclonal antibody targeting programmed death 1 (PD-1) with high specificity and affinity. The programmed death-ligand 1 (PDL-1) expression on tumour cells leads to the evasion of tumour cell escape mechanisms, and allows them to be targeted by the immune cells, which helps to achieve better tumour cell kill. PD-1 receptors are also expressed on CD3+ T cells, and pharmacokinetic studies have demonstrated that median PD-1 receptor occupancy on CD3+ T cells in the peripheral blood of patients with melanoma, treated at a dose from 0.1–10.0 mg/kg every 2 weeks, averaged around 65% for every dose level over 0.3 mg/kg, independent of nivolumab concentrations. Moreover, no correlation between dose, adverse events, and efficacy was observed in clinical trials of anti-PD-1 antibodies across a range of solid malignancies.8-10
Rationale
The introduction of nivolumab has changed the landscape of R/R classical HL treatment, as well as in many other solid tumours like oral cancers, lung cancers (non-small cell lung cancer), renal cell cancers, and melanomas. Despite its effectiveness in many cancers, including R/R HL, its use has been limited, especially in developing countries, due to the prohibitive cost involved. The recommended dose, as per the clinical trial data, is 3 mg/kg (240 mg flat dose) every 15 days, which incurs a huge financial burden to patients, costing around 480,000 INR (5,800 USD) per month.
The FDA approved the therapy for treating adult patients with R/R classical HL after ASCT and treatment with BV.
CASE PRESENTATION
A 21-year-old female was diagnosed with HL and was treated with a doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) regimen. She had a complete metabolic response in interim PET-CT, and progressed after four cycles of an ABVD regimen. The patient then received six cycles of gemcitabine, doxorubicin, and prednisone salvage chemotherapy. She had achieved partial response after three cycles. Despite this treatment, the patient showed signs of relapsed disease, evidenced by the increased size and metabolic activity of the pre-vascular retrosternal lymph nodes, as well as a Deauville score of 5 on the post-treatment (six cycles of gemcitabine, doxorubicin, and prednisone) PET-CT scan (Figure 1).
She had relapsed despite two chemotherapy regimens, so she was started on single-agent low-dose nivolumab (40 mg every 15 days for two cycles). She then underwent fertility preservation prior to transplant as she was still unmarried and planned for ASCT. Her PET-CT after 2 months of nivolumab (Figure 1) showed that she achieved CR, and she was then taken up for ASCT, which was followed by low-dose maintenance for 1 year. She is currently 6 months post-transplant and has been in complete remission based on a PET-CT after 3 months.
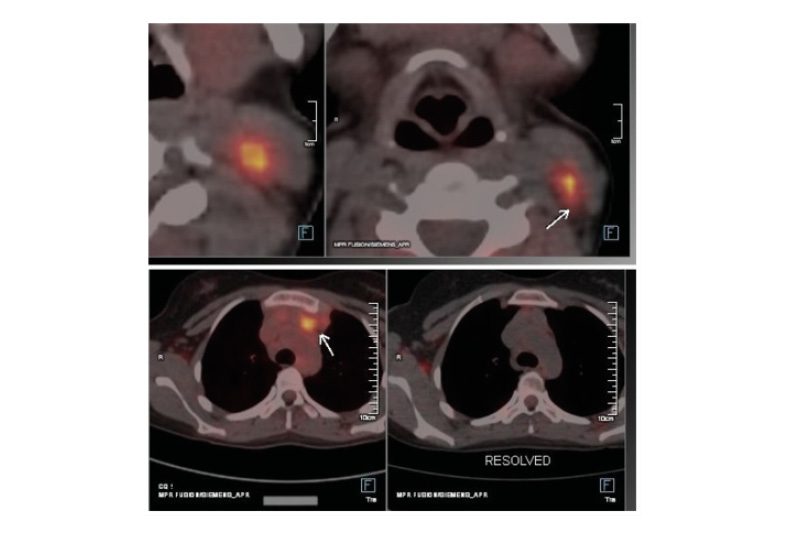
Figure 1: PET-CT image of relapse and response to nivolumab.
Left: increased size and metabolic activity of previously seen prevascular retrosternal nodes (left prevascular node: 33×21 mm; SUVmax: 10.21 [previously 17×13 mm; SUVmax: 5.96]).
Increase in size and uptake of the neck nodes (FDG avid: 13 mm; SUVmax: 10.77 [previously 9 mm; SUVmax: 2.6]). No other new lesions were detected. Deauville score of 5, indicating that the relapsed disease was highly active and required further treatment.
Right: after 2 cycles of nivolumab, there is metabolic resolution of the majority of mediastinal nodes and cervical nodes. Non-FDG avid nodes are at both sites.
FDG: fludeoxyglucose; SUVmax: maximum standardised uptake value.
DISCUSSION
R/R HL is associated with poor outcomes compared to patients who have achieved remission status. Patients with R/R HL have conventionally been treated with salvage chemotherapy, which involves high doses and high toxicity, resulting in significant Grade II–IV cytopenia in 30–45% of patients. Febrile neutropenia in such patients has resulted in considerable morbidity and mortality, as well as the emergence of drug resistance infections.
Salvage chemotherapies were the standard for decades before the advent of genetic tests to know more about the tumour microenvironment. These have specifically demonstrated high PD-1 ligand expression on the surface of Reed-Sternberg cells. There has also been much interest in immunotherapy approaches, particularly PD-1 inhibitors in HL, after initial work in melanomas. This also led to interest in the expression of PD-1 and PDL-1 in oral, lung, and renal cell cancers.
Excellent results were shown from the interim analysis of the SWOG 1826 trial comparing BV, doxorubicin, vinblastine, and dacarbazine (BV-AVD) to nivolumab AVD (N-AVD), with preliminary data suggesting the superiority of N-AVD. The results of clinical trials on the anti-PD-1 antibodies nivolumab and pembrolizumab demonstrated a 1-year overall survival (OS) of 92.0% and a 2-year OS of 91.1%.7-9
While this regimen may change the frontline treatment of advanced-stage HL in developed countries, the prohibitive costs of checkpoint inhibitors at approved doses/schedules make this approach out of reach for most of the world’s population. Despite its efficacy in many cancers, including R/R HL, its use has been limited, especially in developing countries, due to the prohibitive cost involved.
An alternative strategy would be the use of checkpoint inhibitors at low doses. This has never been done in a Phase I trial, and would be based on case reports or post-marketing clinical trials performed only in developing countries. This is because the majority of the population cannot afford the ideal dosing schedules followed/recommended in Western data.
Due to their unique mechanism of action, the efficacy and toxicity profiles of immune checkpoint inhibitors differ from those of chemotherapy and immunoconjugates. The treatment of R/R HL has advanced significantly with the advent of immunotherapy, particularly nivolumab and brentuximab. However, this therapy is only approved for the treatment of adult patients with R/R classical HL after ASCT and treatment with BV.11
Lepik et al.12 showed that a 40 mg flat dose is enough to give non-inferior responses in circumstances where there are resource constraints. They used a 40 mg flat dose of nivolumab every 2 weeks in 30 patients with R/R classical HL, with an OR rate of 70%, 43.3% (13) patients achieving CR, and a median PFS of 18.4 months (95% CI: 11.3–18.5 months).12 The median dose of nivolumab per kg of body weight was 0.59 mg/kg (0.4–1.0 mg/kg).
As per data published by Tata Memorial Hospital, Mumbai, India, in the Indian scenario, around 20–22% of transplant-eligible patients undergo bone marrow transplant, and only a few have access to immunotherapy in full dose.13,14 Many fail to achieve CR after salvage chemotherapy, and are made ineligible for transplant due to infections and complications. For instance, around 30–56% of people develop Grade 3/4 haematological toxicity, and 30–45% develop febrile neutropenia resulting in a significant number of fatalities.13
The LoNAH trial (CTRI/2024/03/063942 [Registered: 11/03/2024])11 is a randomised Phase III study involving patients with newly diagnosed, advanced-stage HL (Stage IIBX, III, or IV). It compares ABVD with a new regimen of n-AVD (comprising low-dose nivolumab [flat dose of 40 mg] + AVD) using a risk-adapted strategy. This trial is currently ongoing, and its results are awaited. This trial may be set up using low-dose AVD as the first-line standard for patients with bulky lymphoma and patients with Stage III/IV lymphoma.15
Relapsed/primary progressive HL responds well to a short course of nivolumab, permitting consolidation with stem cell transplantation. In a study comparing low-dose to standard-dose, the standard dose nivolumab showed no benefit over a dose of 40 mg (0.6 mg/kg), with non-inferior response rates and PFS in both groups.16
There are also data on using nivolumab maintenance for patients with HL who are at high risk of relapse or progression. Sixty percent of patients completed 6 months of maintenance therapy. Six-month PFS was 92.1%, 1-year OS was 100.0%, and 46.0% of patients had adverse events such as thyroiditis, rash, vomiting, or fatigue, with only two patients having pneumonitis or rhabdomyolysis. However, there were no therapy-related deaths.17
The PD1-PD-L1 pathway may facilitate the T cell-mediated tissue damage responsible for graft-versus-host disease (GvHD). Currently, there are a series of publications on GvHD showing that post-exposure to PD-1 blockade agents like nivolumab and pembrolizumab can cause auto-GvHD.18-20 Moreover, engraftment syndrome and auto-GvHD have similar clinical manifestations and exhibit indistinguishable pathological findings on skin biopsy. Auto-GvHD may fall within the same spectrum as engraftment syndrome as an autoinflammatory consequence of breakdown in self-tolerance after ASCT.18
In this case, the patient is an ideal candidate for transplant, but she would not have proceeded to transplant had she not received single-agent low-dose nivolumab. The cost of her therapy was reduced to one-third, and she could save this cost for transplant after achieving CR. She was given nivolumab every 15 days for 2 months before achieving CR, and could achieve fertility preservation prior to transplant. After this, she received maintenance and is in remission post-transplant. So far, the patient has been on nivolumab maintenance therapy for 6 months and does not have any therapy-related side effects or progression/relapse of disease.
This therapy can be used as a bridge to transplant for many transplant-eligible patients, saving a lot of financial resources. It could become the standard of care instead of the toxic salvage regimen, in which one-third to one-half of patients become ineligible due to morbidity or have infection-related mortality.
CONCLUSION
Low-dose nivolumab is a promising therapeutic option for patients with R/R HL, particularly after failure of multiple rounds of chemotherapy. This case highlights the importance of personalised treatment approaches and careful monitoring in achieving optimal outcomes.
This may become a standard, affordable, and effective option, giving patients with lymphoma a chance at cure. It opens up a possible option of low-dose nivolumab for patients with R/R HL as a bridgeto transplant.
This study needs to be validated in larger populations, and the role of nivolumab in the management of R/R HL will continue to evolve, offering hope for patients with otherwise limited options. Future research will help to determine the most effective dosing strategies, the duration of therapy, and the role of nivolumab in combination with other therapies for enhanced outcomes in HL treatment.







