BACKGROUND AND AIMS
Sickle cell disease (SCD) is a genetic disorder characterised by chronic inflammation, vaso-occlusion, and endothelial dysfunction, leading to ischaemic injury and progressive organ damage,1,2 including in the gut.3 Intestinal blood flow impairment in SCD may disrupt gut homeostasis, contributing to dysbiosis, increased permeability, microbial translocation, and systemic inflammation.3-5 This creates a vicious cycle that exacerbates vascular and immune complications.5 Based on the known vascular and anti-inflammatory effects of hydroxyurea (HU), it is hypothesised that this therapy may help preserve intestinal barrier integrity in SCD.6,7 This study aimed to investigate intestinal perfusion and permeability in mice with SCD, and the effects of HU treatment (the standard treatment for SCD).
METHODS
For this, male and female hemizygous (AS) and homozygous (SS) Townes mice (4 months old) were fasted for 4 hours and 60 mg/100 g body weight of fluorescein isothiocyanate (FITC)-dextran was administered orally (4 kDa). After 3 hours, plasma fluorescence was measured by spectrometry.
To assess mesenteric blood perfusion, mice were anesthetised, and the mesentery was exposed. Real-time laser speckle contrast imaging (PeriCam PSI System®, Perimed, Järfälla, Sweden) was used to measure perfusion levels in intestinal loops and mesenteric vessels. A subset of SS Townes mice was treated with HU (50 mg/kg/day for 6 weeks), or not (control-water) to evaluate its effects on intestinal permeability, mesenteric perfusion, and systemic inflammatory markers.
RESULTS
Plasma FITC-dextran levels were significantly higher in SS mice compared to AS controls, indicating increased intestinal permeability (Figure 1A). Additionally, mesenteric perfusion was significantly reduced in SS mice relative to AS mice (Figure 1B), as shown by laser imaging analysis, suggesting altered intestinal vascular function. Notably, HU treatment led to a marked reduction in intestinal permeability, with plasma FITC-dextran levels in HU-treated SS mice approaching those observed in AS controls (Figure 1A). Furthermore, HU significantly improved mesenteric blood perfusion (Figure 1B), with increased perfusion signals in intestinal loops and mesenteric vessels.
SCD mice showed higher serum levels of lipopolysaccharide-binding protein (LBP, Figure 1C), calprotectin (CALP, Figure 1D), intestinal fatty-acid binding protein (IFABP, [/hl]Figure 1E[/hl]), and IL-17 (Figure 1F) compared with controls. HU significantly reduced calprotectin and IFABP levels, suggesting decreased microbial translocation, improved gut barrier function, and inflammation. Interestingly, IL-17 levels increased with HU treatment. Although classically pro-inflammatory, IL-17 also contributes to epithelial barrier maintenance and the regulation of gut microbiota composition.6
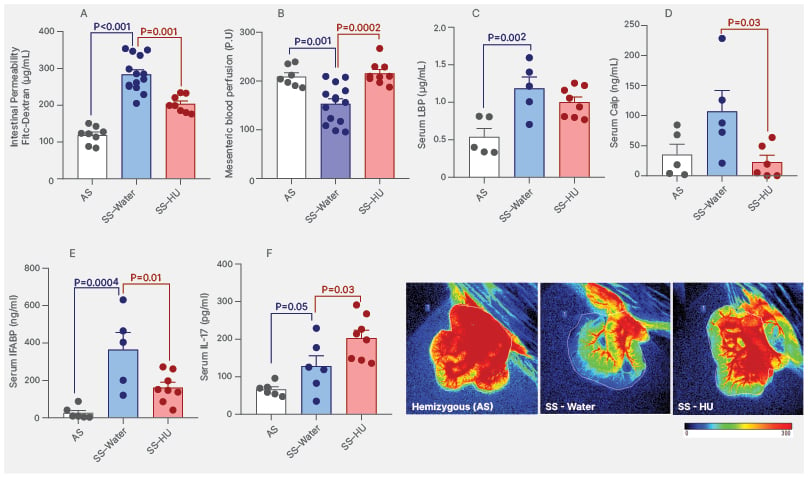
Figure 1: Markers of gut barrier integrity, inflammation, and mesenteric blood perfusion in sickle cell disease mice.
A) Concentration of FITC-dextran in the plasma of AS and SS Townes mice. B) P.U generated from real-time blood perfusion analysis images by laser speckle contrast imaging (PeriCam PSI System®, Perimed, Järfälla, Sweden) in control AS and SS Townes mice. Levels of LBP (C), calp (D), IFABP (E), and IL-17 (F) measured in the serum of Townes mice treated with or without HU. SS Townes mice were treated orally with HU (50 mg/kg/day for 6 weeks) or not (control-water).
The p values represent data from statistical analysis (ANOVA).
Representative images of murine intestinal loops and mesentery circulation were taken during laser speckle contrast imaging to enable real-time assessment of tissue blood perfusion; red colour represents regions with higher blood perfusion.
AS: hemizygous; Calp: calprotectin; FITC: fluorescein isothiocyanate; HU: hydroxyurea; IFABP: intestinal fatty-acid binding protein; LBP: lipopolysaccharide binding protein; P.U: perfusion units; SS: homozygous.
CONCLUSION
In conclusion, the results of this study confirm intestinal dysregulation in SCD mice and indicate that HU contributes to the preservation of gut health. The beneficial effects of HU include reducing systemic inflammation and restoring intestinal barrier integrity, resulting in improved mesenteric and intestinal blood perfusion. These findings suggest that HU, a drug already used in patients with SCD, may also offer a therapeutic strategy for targeting intestinal health management in this population.







