BACKGROUND
TP53 and PPM1D are key regulators of DNA damage response and repair, and somatic mutations in these genes often co-occur in haematopoietic cells, expanding under genotoxic stress. Unlike with TP53 mutations, where mechanisms of progression are defined,1 the pathways underlying clonal fitness and transformation in mutant PPM1D remain poorly defined.2,3
METHODS
In collaboration with five academic institutions, the authors analysed the clinical and molecular landscape of 337 patients with clonal haematopoiesis (CH) and clonal cytopenia of undetermined significance (CCUS) across four genotypes: PPM1D mutations (mt)/TP53-wild type (wt) (n=170 [50%]), PPM1Dmt/TP53mt (n=25 [7%]), TP53mt/PPM1Dwt (n=17 [5%]), and TP53wt/PPM1Dwt (n=125 [38%]).4
RESULTS
All PPM1D variants were truncating, located in exon 6 of the gene, with a median variant allele frequency (VAF) of 6% (0.3–64%). Patients with PPM1Dmt/TP53wt were the oldest (median: 72 years; range: 31–94 years) followed by PPM1Dmt/TP53mt (median: 71 years; range: 52–89 years), PPM1Dwt/TP53wt (median: 69 years; range: 20–99 years), and PPM1Dwt/TP53mt (median: 65 years; range: 52–75 years) groups (p=0.004). Consistent with the notion that DNA damage response gene mutations are preferentially selected under the pressure of anticancer therapy,3 the PPM1Dmt/TP53wt genotype group were most frequent in therapy-related (t) CH/CCUS (80%, 66.5%, 76.5%, and 19%; p<0.001) and had a shorter time interval from last therapy to diagnosis (6.2, 5.9, 11.25, and 24.5 months; p<0.001) compared to PPM1Dmt/TP53wt, TP53mt/PPM1Dwt, and TP53wt/PPM1Dwt, respectively. A significantly higher proportion of patients in the PPM1Dmt/TP53wt (6%/26%) and PPM1Dmt/TP53mt (24%/24%) groups, in comparison to the PPM1Dwt/TP53mt (0%/0%) and PPM1Dwt/TP53wt (0%/3%) groups, received poly ADP-ribose polymerase inhibitors and radioisotope-based cytotoxic therapy, respectively (p=0.01; p<0.001). To describe the position of PPM1D mutations within the clonal architecture, the authors performed a VAF-based analysis to infer clonal hierarchy.5 For this analysis, the authors compared individuals according to disease category and included 58 patients with CH and 137 patients with CCUS. Dominant PPM1D mutations were observed in 32 (53%) patients with CH, and 66 (50%) patients with CCUS (Figure 1A–B).
To further assess whether prior cytotoxic therapy influences the clonal position of PPM1D mutations, the authors stratified each disease group by therapy-related versus sporadic cases, and compared the distribution of dominant, co-dominant, and sub-clonal PPM1D mutations (Figure 1C–D). In the CH subgroup, prior therapy was associated with a higher likelihood of having a dominant PPM1D mutation (56%) than for sporadic cases (28%). The overall distribution of clonal categories differed significantly between therapy-related and sporadic CH (χ²=6.83; degrees of freedom=2; p=0.033; Figure 1C–D). In CCUS, there was no significant difference in clonal status distribution by therapy exposure. Dominant mutations were seen in 44 (48%) therapy-related cases and 22 (53%) sporadic cases, co-dominant in 18 (20%) and five (12%), and sub clonal in 30 (32%) and 14 (34%), respectively (χ²=1.11; degrees of freedom=2; p=0.58).
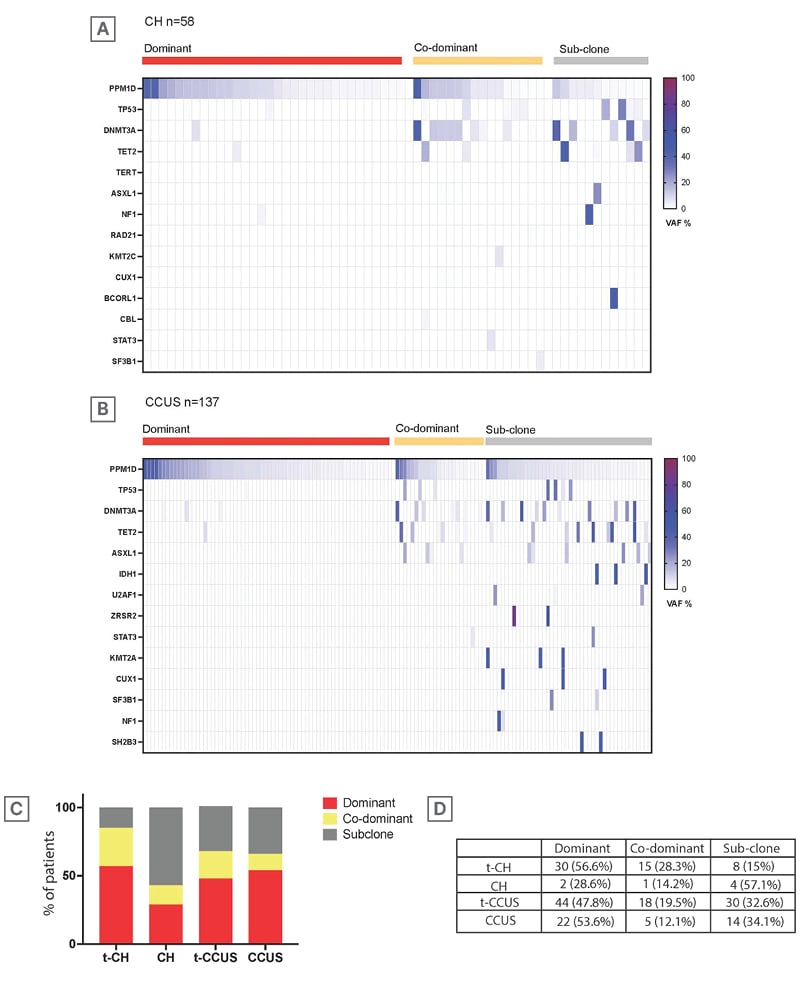
Figure 1: Variant allele frequency-based clonal analysis.
A–B) Heatmaps showing VAFs of somatic mutations in patients with (A) CH (n=58) and (B) CCUS (n=137), categorised by clonal hierarchy: dominant (red), co-dominant (yellow), and sub-clonal (grey).
C) Bar chart comparing the proportion of dominant, co-dominant, and sub-clonal mutations across four groups: t-CH, CH, t-CCUS, and CCUS.
D) Table summarising the percentage of patients within each group who carried dominant, co-dominant, or sub-clonal mutations.
CCUS: clonal cytopenia of undetermined significance; CH: clonal haematopoiesis; t: therapy-related; VAF: variant allele frequency.
CONCLUSION
The authors acknowledge the limitations of their study, including shorter follow-up in the PPM1Dmt/TP53wt and PPM1Dmt/TP53mt groups, as well as heterogeneity in genomic sequencing techniques between the five academic institutions that participated in this study. However, to the best of their knowledge, this work represents the largest series reporting on the spectrum of precancerous PPM1D mutations in the context of evolving cancer therapies. In conclusion, while PPM1D mutations were frequently identified in t-CH/CCUS and associated with unexplained cytopenias in the context of low VAF, within limitations of a short follow-up in our cohort, they were associated with a low rate of myeloid clonal evolution, even in the presence of TP53mt. Longer-term follow-up is planned to better assess impacts on progression free survival and overall survival.







