Abstract
Rituximab, a monoclonal anti-CD20 antibody, has proven to be an excellent therapeutic agent for haematological diseases. Low-dose rituximab (LDR; 100 mg weekly for 4 weeks) has emerged as a promising alternative to the standard dosing (375 mg/m²) for autoimmune haematological disorders, offering comparable efficacy with reduced adverse events and costs. In this review, the authors examine LDR’s role in immune thrombocytopenia, thrombotic thrombocytopenic purpura, and autoimmune haemolytic anaemia (AIHA) in the adult population. For immune thrombocytopenia, studies demonstrate that LDR achieves high overall response rates in both patients with relapsed/refractory disease and those who are newly diagnosed, confirming its non-inferiority to standard dosing. Combination therapies including thrombopoietin agonists further enhance efficacy. Single-dose rituximab shows comparable efficacy to LDR, but reduces indirect costs derived from hospital visits. In thrombotic thrombocytopenic purpura, LDR combined with plasma exchange plus steroids reduces relapse rates, in-hospital stays, and procedures, though prospective data remains limited. For AIHA, LDR combined with steroids or other agents (such as the immunomodulatory drug, bortezomib) induces high response rates, particularly in warm AIHA. Overall, LDR exhibits favourable safety (with minimal infectious complications) and cost-effectiveness across these disorders. While current evidence supports LDR as a front-line or salvage therapy, further research is needed to optimise dosing, identify ideal candidates, and validate combination strategies in randomised trials.
Key Points
1. Low-dose rituximab (LDR) has been shown to be an effective treatment for autoimmune haematological disorders such as immune thrombocytopenia, thrombotic thrombocytopenic purpura, and autoimmune haemolytic anaemia, with comparable efficacy to standard doses and fewer adverse effects.2. LDR, when used in combination with other therapies, has demonstrated improved outcomes in both patients with relapsed disease and those who are newly diagnosed. LDR combined with eltrombopag or romiplostim has shown enhanced efficacy in immune thrombocytopenia, and its use in combination with dexamethasone in autoimmune haemolytic anaemia has led to high response rates.
3. Despite its promising results, more prospective studies are required to fully establish LDR’s role in the treatment of autoimmune haematological disorders.
INTRODUCTION
Rituximab, a monoclonal anti-CD20 antibody, has become the second-line treatment for autoimmune haematological disorders such as immune thrombocytopenia (ITP), thrombotic thrombocytopenic purpura (TTP), and autoimmune haemolytic anaemia (AIHA).1 Both peripheral and bone marrow B lymphocytes are depleted by rituximab, reducing the autoreactive B cell burden in autoimmune disease.2,3
According to the American Society of Hematology (ASH), the standard dose regimen (SDR) of rituximab is 375 mg/m2 weekly for 4 weeks, originally approved for the treatment of Non-Hodgkin’s lymphoma. This standard dosing approach has achieved a 69% overall response rate (ORR) and a 35% sustained response rate in relapsed or refractory (R/R) ITP. In acute TTP, standard of care (SOC) plus rituximab reduced inpatient stay and relapse.4,5 Relapse-free survival (RFS) was superior after a combined therapy including rituximab in the SDR, rather than monotherapy with steroids in AIHA.
Aiming to reduce rituximab-related adverse events, infusion time, and costs whilst maintaining efficacy, a reduced dosing schedule (100 mg weekly for 4 weeks) has been proposed for autoimmune haematological disorders.6 Therefore, this review explores the evidence supporting the efficacy and safety of low-dose rituximab (LDR) for the treatment of ITP, TTP, and AIHA.
IMMUNE THROMBOCYTOPENIA
ITP is characterised by isolated thrombocytopenia due to autoantibody-mediated platelet destruction in the spleen and liver.7 Notably, children with ITP tend to experience spontaneous remission within months. On the contrary, up to 70% of adults diagnosed with ITP will continue to have a chronic condition with recurrence and will require further therapy lines.8 The goal of therapy is to prevent bleeding through immunomodulation of the pathological B cells. Although steroids in first-line treatment provide a high initial response, only 30–50% of patients achieve a sustained response (SR).8 LDR in combination with steroids has demonstrated superior outcomes compared to the latter alone.9 Therefore, LDR is a potential therapy line for patients with relapsed disease.
A small, single-arm study reported an ORR and complete response (CR) rate of 75% and 43%, respectively, in 28 patients with R/R ITP who were treated with LDR. These are comparable rates to the SDR.10 Subsequently, LDR therapy was confirmed to be effective in an additional cohort of 48 patients with previously treated ITP. The ORR and CR were 60.5% and 39.5%, respectively, with an RFS at 24 months of 45%. Of note, relapse was more probable in patients with a higher weight and those who experienced a longer time interval from diagnosis to treatment.11 These Phase II studies demonstrated the utility of LDR as a salvage therapy for ITP, as well as its limitations, such as inefficacy related to body weight. Only one infectious complication was reported.11 Retrospective real-world studies further support the use of LDR in the salvage setting.6,12
In the front-line setting, a single-arm trial evaluated the efficacy and safety of LDR in combination with high-dose (HD) steroids (40 mg dexamethasone daily for 4 consecutive days) in 21 patients with newly diagnosed (ND) ITP. Remarkably, the ORR at Day 28 and the CR at 6 months were 90.5% and 76.2%, respectively. The median duration of CR was 17 months. Only 9.5% of patients presented adverse events, including Grade 1 chills (n=1) and Grade 1 flu-like symptoms (n=1). Notably, no infectious complications were reported either.13 In a recent retrospective study in the same centre, consistent ORR and CR at Day 28 (88.2% and 58.8%, respectively) were reported in an additional group of 17 patients with ND ITP.14 Both of these studies proved the superior efficacy of LDR in ND ITP compared to R/R ITP, which suggests that an ‘all in’ strategy should be used in the front-line setting.
In a 2021 meta-analysis, LDR for ITP was compared to standard dose through 12 studies, which included a total of 869 patients. When compared to the control groups, rituximab improved the ORR, CR, and sustained response rate without a significant increase of infectious or other adverse events. No statistically significant differences were found between LDR and the SDR in relation to efficacy.15 These results consolidate LDR’s non-inferiority to the SDR. Consequently, an important reduction in costs while maintaining efficacy is attainable. However, the follow-up periods during the individual studies were short (1.0–19.5 months),15 and therefore long-term infectious complications could be underestimated. A long-term follow up of patients treated with the SDR determined the 2-year serious infectious event rate was 11.3 per 100 person-years, especially in older patients with additional comorbidities.16 A large retrospective study of patients diagnosed with rheumatoid arthritis determined that LDR had statistically lower infectious complications.17
Due to the limitations of LDR, a prospective multicentre, open-label, randomised controlled trial was conducted to compare a single administration of the SDR versus LDR in 94 patients with ITP who relapsed or were corticoid-refractory. The efficacy was not different between the regimens regarding ORR or CR. Nonetheless, single-dose rituximab resulted in a better health-related quality of life and fewer physician visits.18 Trials in paediatric populations have also reported superior benefits from single dosing versus LDR.19 In the future, this regimen may be preferred in some patients for reducing hospital visits. Nonetheless, body weight-related inefficacy has not been described in the single-dose regimen. More research is needed to confirm if this strategy is able to bypass this limitation compared to LDR.
Finally, LDR has been studied in combination with other drug groups. A recent case series of 10 patients with R/R ITP reported the efficacy of eltrombopag in combination with LDR.20 In the front-line setting, 13 patients with ND ITP were included in a single arm, open-label study and received HD dexamethasone, eltrombopag, and LDR, archiving a 100% ORR. The duration of response was 11 months.21 Moreover, the safety and efficacy of romiplostim plus LDR plus HD dexamethasone has been evaluated in a pilot study including 13 patients with ND ITP. The 1-month ORR and CR reported were 92.3% and 84.6%, respectively.22 These studies prove the improvement in efficacy of thrombopoietin receptor agonists in combination with LDR and HD steroids for both R/R ITP and ND ITP. Likewise, a two-arm randomised clinical trial compared LDR ± all-trans retinoic acid (ATRA) in 168 patients with R/R ITP. The addition of ATRA to LDR resulted in an improvement in ORR (80% versus 59%) and sustained response rates (61% versus 41%). However, the combination groups demonstrated a higher incidence of adverse events (70.5% versus 53.6%).23 While LDR-based combination therapies are promising, more trials are needed to establish them as part of the SOC for ITP.
In summary, LDR is an efficacious option for patients with R/R ITP and those with ND ITP, with low rates of complications and lower direct costs (Table 1). Nonetheless, the ideal dosing of rituximab should be determined by further exploration in both sets of patients.
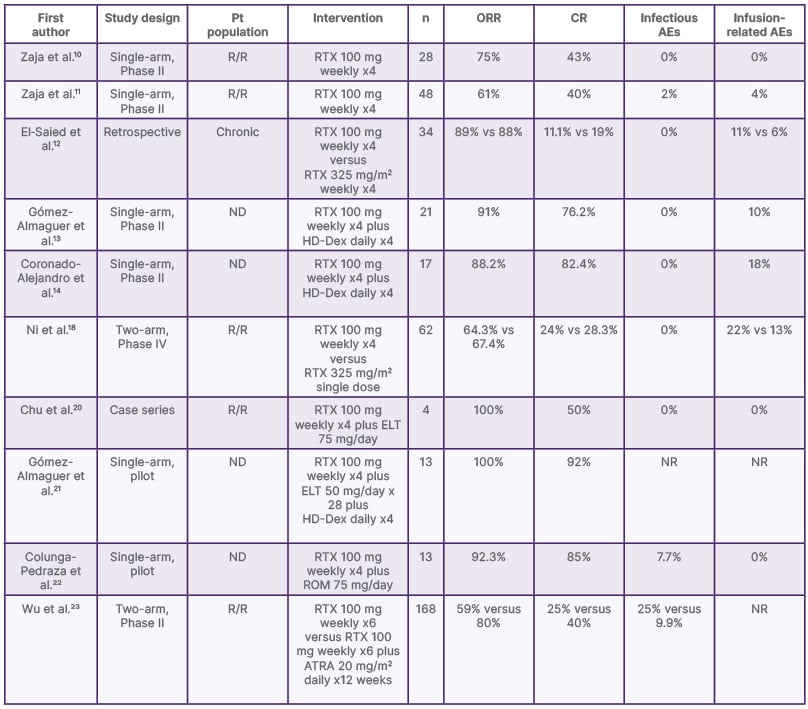
Table 1: Selected low-dose rituximab studies in immune thrombocytopenia.
AE: adverse event; ATRA: all-trans retinoic acid; CR: complete response; ELT: eltrombopag; HD-Dex: high-dose dexamethasone; ND: newly diagnosed; NR: not reported; ORR: overall response rate; Pt: patient; R/R: relapsed or refractory; ROM: romiplostim; RTX: rituximab; vs: versus.
THROMBOTIC THROMBOCYTOPENIC PURPURA
TTP is a thrombotic microangiopathy characterised by the formation of microvascular platelet-rich thrombi, which results in microangiopathic haemolytic anaemia, thrombocytopenia, and end-organ ischaemia.24 Plasma exchange (PEx) is recognised as the first-line treatment in TTP, with rituximab being used preferably in R/R TTP.24 Some studies have assessed the potential of LDR in acute TTP or R/R TTP (Table 2).
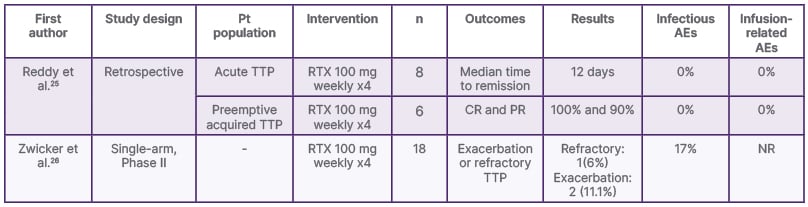
Table 2: Selected low-dose rituximab studies in thrombotic thrombocytopenic purpura.
AE: adverse events; CR: complete response; NR: not reported; PR: partial response; Pt: patient; RTX: rituximab; TTP: thrombotic thrombocytopenic purpura.
In 2013, Pequeño-Luévano et al.27 successfully treated four patients with acute TTP using LDR, PEx, and a short course of steroids with an excellent outcome.27 In another case series, two patients with systemic lupus erythematosus who presented with TTP reported successful therapy with LDR in combination with PEx. Neither had TTP relapses or systemic lupus erythematosus flares within the 24-month follow-up.28 In a third case series of two patients with refractory TTP treated with LDR, the patients had a sustained long-term remission for 19 and 23 months, respectively.29
Reddy et al.25 performed a retrospective analysis over 13 years of 39 patients with TTP who were treated with either 100 mg weekly (2–4 doses) or 375 mg/m2 weekly (2–4 doses). Time to remission from the first rituximab dose was 6 days in patients receiving the standard regime and 5 days in patients treated with LDR (p=0.09). Eleven PEx procedures were performed in the standard dose group versus eight in the low dose group (p=0.206). Length of stay was shorter in the lower dose group (p=0.023).25
Due to the lack of prospective studies evaluating the efficacy and safety of LDR in the treatment of TTP, a clinical trial was performed by Zwicker et al.26 in 2019. The trial reported that, in patients with acute TTP and ADAMTS13 activity of <10%, LDR along with PEx and corticosteroids was associated with a significant decrease in the incidence of exacerbation and refractory TTP.26 The results of this study establish that LDR can be used in the immediate treatment of TTP.26
Due to a lack of consistent evidence, more prospective studies evaluating LDR in the context of TTP are needed. With the current evidence, LDR cannot currently be securely integrated into the SOC. Other therapeutic alternatives have recently emerged, such as caplacizumab, a nanobody directed against the A1 domain of von Willebrand factor. This agent, in combination with therapeutic plasma exchange and corticosteroids, has shown to reduce mortality, relapse rates, and therapeutic plasma exchange in patients with TTP, although it may be associated with adverse effects such as mucosal bleeding.30-32 Both caplacizumab and rituximab are complementary agents with potential utility during acute episodes, achieving a more rapid clinical response when used in combination (14 days) compared to treatment with rituximab alone (24 days).33
AUTOIMMUNE HAEMOLYTIC ANAEMIA
Rituximab has emerged as an alternative treatment in patients with R/R AIHA, milder disease, or a high risk of side effects due to standard dose. To date, steroids have been the first-line treatment option for patients with AIHA. Several studies have analysed other interventions such as rituximab, alemtuzumab, cyclophosphamide, and bortezomib along with steroids. Some studies have even tested LDR in ND warm AIHA (wAIHA)34-37 (Table 3). Standard and low doses of rituximab have shown to improve haemoglobin levels and reduce the need for blood transfusion in these patients.
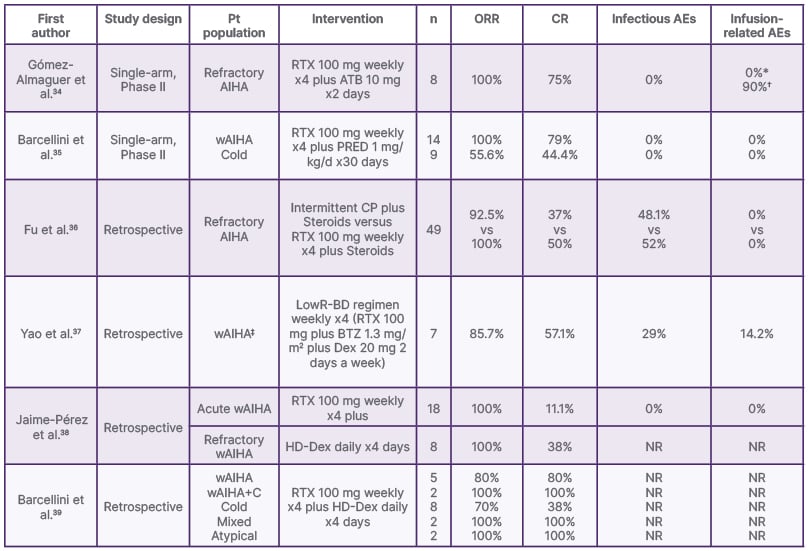
Table 3: Selected low-dose rituximab studies in autoimmune haemolytic anaemia.
*No infusion-related adverse events with the rituximab.
?Grade 1 fever after alemtuzumab administration.
‡Subtype not specified.
AE: adverse event; AIHA: autoimmune haemolytic anaemia; ATB: alemtuzumab; BTZ: bortezomib; CP: cyclophosphamide; CR: complete response; Dex: dexamethasone; HD-Dex: high-dose dexamethasone; LowR-BD: low dose rituximab-bortezomib and dexamethasone; NR: not reported; ORR: overall response rate; PRED: prednisone; Pt: patient; RTX: rituximab; wAIHA: warm autoimmune haemolytic anaemia; wAIHA+C: warm autoimmune haemolytic anaemia IgG plus C3d.
A retrospective cohort containing 18 patients with newly diagnosed wAIHA treated with LDR and HD dexamethasone as a front-line therapy reported an RFS at 6, 36, and 72 months of 92.3%, 58.7%, and 44.1%, respectively. All patients achieved a response, and there was no statistical difference in RFS between patients treated with steroids and those treated with LDR plus steroids as a front-line treatment. Eight patients were treated with LDR and HD dexamethasone after the diagnosis of refractory wAIHA. All of the patients achieved a response (three complete responses and five partial responses).38
The GIMEMA study included 308 patients with AIHA, 19 of whom received LDR as a second or third-line therapy. The intervention induced a CR of 100% in patients with wAIHA IgG plus C3d (C3d complement), mixed and atypical. Patients with wAIHA achieved a CR of 80%, followed by a CR of 37.5% and a partial response (PR) of 37.5% in cold agglutinin disease. Predictors of response to LDR were wAIHA, younger age, and a shorter interval of time between the diagnosis and rituximab therapy.39
LDR in combination with alemtuzumab has proven to be safe and capable of inducing an important clinical response in patients with active symptomatic steroid-refractory AIHA. Gómez-Almaguer et al.34 evaluated the safety and efficacy of this combined regimen in refractory AIHA. ORR was 100% and CR was 75%. Patients in this regime had a median duration of response of 46 weeks.34
A monocentric retrospective study including 49 patients with refractory AIHA was performed in 2016, and it compared the efficacy of intermittent cyclophosphamide IV plus steroids in one group, as well as LDR plus steroids in a second group. Patients receiving intermittent cyclophosphamide IV plus steroids achieved an ORR of 92.5% and a CR of 37%. Patients in the LDR plus steroids group achieved an ORR of 100% and a CR of 50%. LDR treatment seemed to have a quicker and greater effect on the patients than cyclophosphamide.36
In 2022, a retrospective study with seven patients evaluated the efficacy of LDR with dexamethasone and bortezomib to treat wAIHA. The ORR was 85.71%, with a PR of 57.41% and a CR of 28.75%. They demonstrated that LDR combined with bortezomib and dexamethasone is effective and relatively safe in patients with wAIHA.37
Given the lack of standardisation of the rituximab doses to be used in autoimmune diseases such as AIHA, Moser et al.40 performed a Phase II open-label trial in 2024 that investigated the effects and safety of “very” LDR, which range from 5 mg/m2 to 100 mg in patients with AIHA. Rituximab doses as low as 5 mg/m2 transiently depleted CD20+ cells in almost all patients, but the tested low-dose regimens failed to permanently suppress CD20+ cells.40
In 2021, a Phase II single-arm trial including 23 patients evaluated oral prednisone plus LDR as a first-line therapy in ND wAIHA and CAD, and as a second-line therapy in wAIHA that had relapsed after standard oral prednisone. Patients with wAIHA accomplished an SR of 100%, a CR of 69.2%, and a PR of 20.8% at 12 months, as well as a relapse rate of 0%. In CAD, an SR of 50%, a CR of 16.6%, and a PR of 33.3% were achieved, with a relapse rate of 33.3% at 12 months. This study concluded that LDR in addition to a short course of steroid therapy is a safe and effective treatment, particularly in wAIHA and in patients with newly diagnosed disease. Nonetheless, in CAD, the risk of relapse was higher.35 A 10 year follow-up update reaffirmed the efficacy and safety of LDR in primary AIHA as a short-term response and long-term outcome. It was demonstrated that LDR is more effective and leads to an SR in wAIHA compared to CAD.41
CONCLUSION
LDR is an effective treatment in both front-line and salvage settings in autoimmune haematological diseases. Few adverse events are related to LDR, with very low infectious complications. Furthermore, LDR has been safely added to combination regimens. In the future, LDR may be established as a relevant option for front-line treatment in both haematological42 and not-haematological immune-mediated pathologies.43 Nevertheless, as exemplified by TTP, more trials are needed before it can be safely integrated into clinical practice. Currently, studies with small samples predominate, and thus cannot powerfully determine how LDR could fit into the SOC. Clinically relevant questions, such as whether LDR reduces the incidence of late-onset neutropenia or decreases the need for intravenous or subcutaneous Ig, remain unanswered due to limited evidence, and warrant further investigation. Future research, with greater sample sizes and controlled designs, should focus on the specific patient subgroups that could benefit the most from LDR combinations.







