BACKGROUND AND AIMS
The liver exhibits metabolic zonation, with gene expression and metabolic functions distributed across specific zones along the lobule radial axis.1,2 Disruption of zonation is linked to the initiation and progression of liver pathologies. Neddylation, a post-translational modification essential for metabolism, influences zonal metabolic processes and is associated with liver pathogenesis.3 However, its relationship with liver zonation remains understudied. This study aims to investigate the connection between neddylation and liver zonation, evaluating its metabolic implications and role in liver diseases.
METHOD
Spatial transcriptomics was used to assess the localisation of neddylation machinery gene expression in mice. Biotinylated ubiquitin and biotinylated NEDD8 mice were employed to evaluate post-translational modifications in known zonated proteins. The authors also utilised different transgenic mouse models to study: 1) the impact of zonation loss on neddylation using LiTSC1KO RagAGTP mutants,4 which exhibit zonation disruption without hepatic disease at early stages; and 2) the role of neddylation modulation on zonation, using models that silence or overexpress NEDD8, silence NAE1 (a key NEDD8 activator), or overexpress SENP8 (a major deneddylation protease).
RESULTS
Neddylation genes were more highly expressed in pericentral hepatocytes than periportal ones. Proteomic data from biotinylated NEDD8 and biotinylated ubiquitin mice revealed neddylation and ubiquitination enrichment on periportal zonation markers (e.g., ALB, ASS, ASL, CYP2F2). In LiTSC1KO RagAGTP mutant mice, zonation loss altered the expression of neddylation mediators. Immunofluorescence showed a decentralised pattern of NEDD8 in the mutants compared to its periportal localisation in controls. Silencing NAE1 or overexpressing SENP8 (which encodes the deneddylase NEDP1) disrupted periportal marker gene expression, while NEDD8 modulation also affected central zone markers’ mRNA levels. Mutant models showed altered expression of genes involved in zonated metabolic processes, with pronounced effects when NEDD8 or NAE1 were manipulated.
CONCLUSION
The results demonstrate a significant association between neddylation and liver zonation, suggesting a zonal expression pattern of the neddylation machinery. Genetic manipulation of neddylation mediators influenced zonal markers and altered the expression of genes involved in zonated metabolic processes, reinforcing the link between neddylation and liver zonation (Figure 1). Future research will explore zonal neddylation modulation to elucidate its role in liver metabolism and pathology.
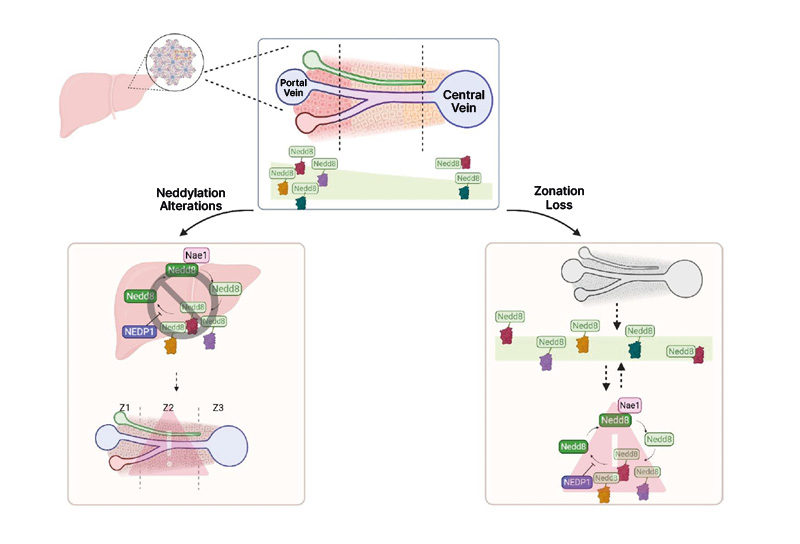
Figure 1: Schematic representation of the relationship between neddylation and liver zonation.







