Abstract
Percutaneous coronary intervention has evolved significantly due to advancements in interventional strategies and device technologies. Drug-coated balloons (DCB) have emerged as drug delivery devices that don’t use a metallic scaffold, delivering antiproliferative drugs directly to the arterial wall. Paclitaxel-coated balloons have demonstrated efficacy in treating in-stent restenosis and small vessel disease; however, there is still limited evidence regarding their efficacy and safety in treating bifurcation and large vessel de novo lesions. Sirolimus-coated balloons (SCB) have shown promising outcomes in preclinical studies, exhibiting reduced smooth muscle cell loss and lower downstream embolisation. SCBs have demonstrated efficacy in treating in-stent restenosis and small vessel disease in clinical trials, but long-term outcome studies remain limited. This review explores preclinical and clinical data on DCBs, highlighting the vascular response, pharmacokinetics, and comparison of effectiveness between paclitaxel-coated balloons and SCBs. Furthermore, novel technologies, including everolimus and dual-drug formulations, are being investigated to enhance therapeutic outcomes. While DCBs provide a viable alternative to drug-eluting stents for specific indications, further research is needed to establish optimal patient selection, refine drug delivery mechanisms, and evaluate long-term outcomes. The evolution of DCBs continues to shape future percutaneous coronary intervention strategies, potentially offering a scaffold-free approach with equivalent or improved clinical outcomes.
Key Points
1.Drug-coated balloons provide an approach to percutaneous coronary intervention that doesn’t involve a metallic scaffold by delivering antiproliferative drugs directly to the arterial wall. This prevents restenosis and preserves future treatment options. Emerging data suggest that the balloons may be a viable alternative to drugeluting stents, especially for the treatment of in-stent restenosis, small vessel disease, and bifurcation disease.
2. Paclitaxel-coated balloons were the first drug-coated balloons used in clinical practice. They are primarily composed of an excipient, meant to enhance delivery, and the antiproliferative drug paclitaxel. First-generation paclitaxel-coated balloons mainly contain a dehydrated crystalline coating, which promotes drug retention and extends bioavailability, preventing restenosis. However, this design also generates substantial particulates of varying size, and may be associated with downstream emboli and tissue injury to non-target organs.
3. Sirolimus-coated balloons (SCB) offer the potential of well-tolerated cytostatic agents, but require the use of drug carriers to prolong arterial wall levels. In experimental models, SCBs are associated with fewer embolic non-target organ effects and decreased tissue injury. While SCBs have demonstrated efficacy in treating in-stent restenosis and small vessel disease, larger outcome studies remain limited. Larger, better-designed, and definitive trials are needed.
INTRODUCTION
Percutaneous coronary intervention (PCI) has achieved remarkable progress due to advances in technology, including the devices themselves, ancillary equipment, and pharmacological therapies. Although balloon angioplasty initially proved effective in restoring blood flow, it was associated with restenosis in 50–60% of patients within a year post-procedure due to vessel recoil, constrictive remodelling, and neointimal proliferation.1,2 Advances in stent technology have effectively addressed these issues, and second-generation drug-eluting stents (DES) have become the gold standard for the treatment of coronary artery disease (CAD). However, clinical studies have indicated that DESs are associated with an increased risk of late events and neoatherosclerosis, with approximately 2% of patients annually experiencing very late ischaemic events, and ischaemia-driven target lesion revascularisation (TLR) occurring in 4.4% of subjects during a follow-up period of 1–5 years.3,4 Furthermore, leaving a permanent metallic scaffold may not be an optimal strategy for complex lesions and could limit future treatment options.
Drug-coated balloons (DCB) have recently attracted attention as a novel interventional therapy.5 The primary advantage of this technology lies in its ability to transfer antiproliferative agents to the luminal surface of the vessel without requiring permanent scaffolds. Numerous preclinical studies, which primarily utilise crystalline formulations of paclitaxel-coated balloons (PCB) in animal models, have evaluated the biological response of the vasculature to the drug and the safety of DCB therapy.6-9 Medial smooth muscle cell (SMC) loss and detectable particulate debris in downstream organs are hallmarks of this technology, with clinical consequences that are currently unclear. With continuous advancements in DCB technology, sirolimus-coated balloons (SCB) have more recently emerged as a promising alternative. Two of the most advanced programs, the MagicTouch™ (MT)-SCB (Concept Medical, Tampa, Florida, USA) and SELUTION SLR™ (SEL)-SCB (MedAlliance, Nyon, Switzerland), which both use microcarriers, have been reported to induce minimal arterial injury and downstream effects.10 Furthermore, clinical data have demonstrated the efficacy and safety of these and other SCBs in small vessel disease (SVD) and in-stent restenosis (ISR),11,12 although larger studies are needed and are currently underway. In this review, the authors comprehensively examine the role of DCBs in PCI from both preclinical and clinical perspectives.
MECHANISTIC UNDERPINNINGS OF DCBS
The primary mechanisms underlying late luminal loss following plain old balloon angioplasty (POBA) include the formation of a fibrocellular neointima at the injury site and negative arterial remodeling.13 Mechanical stress induced by POBA disrupts endothelial cells and the medial wall, leading to restenosis. This triggers a cascade of repair mechanisms involving platelet-fibrin deposition, inflammation, growth factor release, SMC proliferation, and extracellular matrix deposition. The activation of cell cycle proteins and mitosis constitutes a final common pathway, ultimately resulting in neointimal proliferation.14
As there is no scaffold from which to elute antiproliferative agents, the key to DCB success lies in its ability to efficiently and safely transfer the drug into the vascular tissue. The drug must also be able to sustain its therapeutic effects for an adequate duration. Unlike DESs, the transfer of the drug must take place during the initial inflation. The two principal drugs utilised in DCBs are paclitaxel and sirolimus, both of which act as antiproliferative agents by inhibiting SMC proliferation, but work by a distinct mechanism (Figure 1).
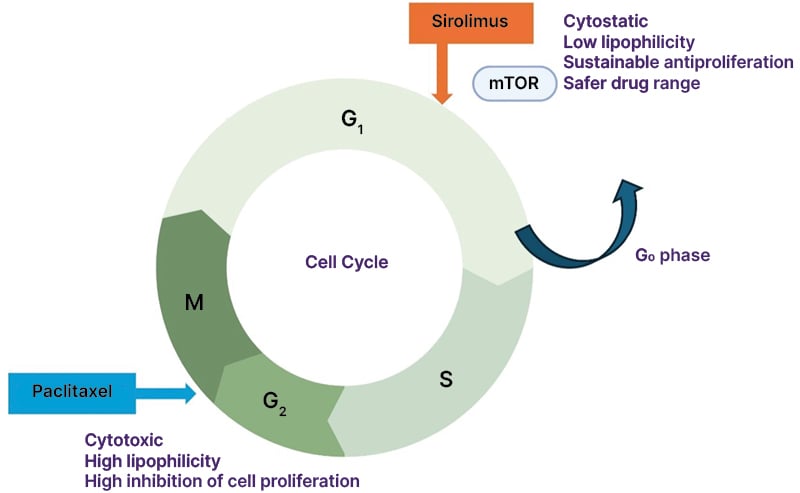
Figure 1: The features of sirolimus and paclitaxel.
The increased stability of microtubules in the presence of paclitaxel at G2/M phases results in cell apoptosis (cytotoxic), leading to the inhibition of neointimal hyperplasia. Paclitaxel has high lipophilicity and tissue affinity, inhibiting cell proliferation. Sirolimus (rapamycin) inhibits the G1/S cell cycle transition by forming a complex with FKBP12 (FK506-binding protein 12), which then binds to and inhibits the mammalian target of rapamycin. This reversibly induces cells to enter the G0 quiescent phase (cytostatic), resulting in a potent and sustainable antiproliferative effect. Unlike paclitaxel, sirolimus has low lipophilicity and tissue affinity.
Paclitaxel stabilises microtubules and arrests the cell cycle at the G2/M phase, leading to cell death,15,16 which thereby suppresses neointimal hyperplasia, a key contributor to restenosis. The physical state of solid-phase paclitaxel on the balloon surface varies from amorphous (non-crystalline) to crystalline forms.17 Amorphous paclitaxel exhibits high vascular adherence and facilitates effective drug transfer into the arterial wall, but has limited long-term retention and bioavailability. In contrast, crystalline paclitaxel demonstrates a slower dissolution rate, leading to prolonged drug bioavailability and sustained biological effects.18 By optimising the balance between these properties and selecting appropriate excipients, passive absorption and retention of the drug within the arterial wall can be achieved, ensuring sustained therapeutic concentrations that prolong the antiproliferative effect.6 Despite this, most commercially available paclitaxel DCBs utilise crystalline paclitaxel as a primary ingredient, which helps improve tissue drug retention.
Sirolimus exerts its potent, sustainable antiproliferative effects by reversibly binding to FKBP12, forming a complex with the mammalian target of rapamycin and inhibiting the G1/S cell cycle transition, which reversibly induces cells to enter the G0 quiescent phase.19, 20 Compared to paclitaxel, sirolimus exhibits superior anti-restenotic and anti-inflammatory properties, which is one of the reasons why paclitaxel-eluting stents are no longer used for patients with CAD.21 However, since tissue absorption of sirolimus is lower than that of paclitaxel, drug transfer necessitates the development of innovative excipients and carriers to optimise drug delivery for SCB treatment. Advanced formulation strategies enhance drug retention and bioavailability within the arterial wall. To overcome these issues, microcarriers are used for SCBs to facilitate drug dissolution over time. MT-SCB uses phospholipids comprising one hydrophilic head and two lipophilic tails, improving the adhesion properties of encapsulated sirolimus.22 Microreservoirs in the SEL-SCB are advanced drug-delivery systems composed of a biodegradable polymer (i.e., poly[lactic-co-glycolic acid]) intermixed with sirolimus, ensuring consistent and predictable drug release for up to 90 days.23 Furthermore, Cell Adherent Technology (CAT™ [MedAlliance, Nyon, Switzerland) enhances drug retention and bioavailability by securely binding these microreservoirs to the balloon surface, enabling a lower drug dose while optimising sirolimus transfer and uptake.24 These advances in microparticle and nanoparticle technologies have the potential to improve drug delivery and reduce embolic risks. Newer experimental systems, such as those from Advanced NanoTherapies (Santa Clara, California, USA), use nanoparticle carriers to encapsulate sirolimus and paclitaxel (bench testing of the Philips Stellarex™ DCB [Amsterdam, the Netherlands], the Becton Dickinson Lutonix™ DCB [Franklin Lakes, New Jersey, USA], and the Advanced NanoTherapies SirPlux Duo DCB has been performed. Data are on file at Advanced NanoTherapies), but more work is needed to explore the safety and efficacy of these systems.
PRECLINICAL STUDIES FOR PCBS AND SCBS
DCB Response and Pharmacokinetic Levels in the Treated Area
Animal experiments for DCBs have predominantly been conducted based on the clinical indication sought. These evaluations look for evidence of systemic toxicity, distal emboli, and vascular changes, as well as some indirect measures of efficacy such as histological drug effects and pharmacokinetics. In 2004, Scheller B et al.25 were the first to evaluate differences in neointimal formation following the implantation of a bare-metal stent (BMS) crimped onto either conventional uncoated balloons or onto three types of PCBs differing in drug dose. A histopathological evaluation was performed five weeks after implantation. As a result, DCBs utilising an acetone-based coating demonstrated a significant, dose-dependent reduction in late lumen loss and neointimal area.25 Furthermore, in a preclinical study evaluating plain balloons and PCBs using iopromide or urea as carriers, with dose ranges from 1 to 9 µg/mm² and triple application of balloons coated at the standard dose (3 µg/mm²), the neointimal area in the uncoated control group was 6.8±2.2 mm². In contrast, at a dose of 1 µg/mm², the neointimal area was significantly reduced to 3.1±1.1 mm² with iopromide as the carrier, and to 3.0±0.5 mm² with urea as the carrier. At 3 µg/mm², the neointimal area was further reduced to 2.0±0.4 mm² with iopromide and 1.7±1.1 mm² with urea. This indicates that efficacy was observed even at the lower dose of 1 µg/mm². However, increasing the dose beyond 3 µg/mm² did not yield additional benefits.26 These studies served as early proof of concept supporting the efficacy of DCBs, and stimulated further research in the field.
A preclinical study comparing Lutonix-PCB (Lutonix® 035 [Becton Dickinson]) with POBA for treatment of the superficial femoral artery7 demonstrated that at 28 days post-DCB treatment, no fibrin deposition or endothelial cell loss was observed in the intima, and extensive destruction of the media or external elastic lamina was rarely noted. However, compared to the POBA, Lutonix-PCB exhibited significant inflammation at 28 days, which resolved by 90 days. Additionally, SMC loss, as well as proteoglycan and collagen deposition, was more pronounced in Lutonix-PCB versus POBA at all time points, with the area of SMC loss peaking at 90 days. The extent of adventitial fibrosis progressively increased from 28 to 90 days and persisted up to 180 days, presumably due to paclitaxel-induced high tissue permeability. Moreover, a study comparing histological changes among commercially available DCBs approved for above-the-knee disease6,8 found that at 28 days, the total medial SMC loss score in DCB-treated segments was significantly higher with IN.PACT-PCB (IN.PACT™ Admiral™ [Medtronic, Dublin, Ireland]) as compared to Lutonix-PCB. Localised medial SMC loss was associated with increased proteoglycan accumulation, which was more pronounced in IN.PACT-PCB.6 Among the Ranger-PCB (Ranger™ [Boston Scientific, Marlborough, Massachusetts, USA]), IN.PACT-PCB, and Stellarex-PCB, the depth and circumferential extent of medial SMC loss yielded similar findings.8 Table 1 summarises FDA-approved DCBs for above-the-knee disease.
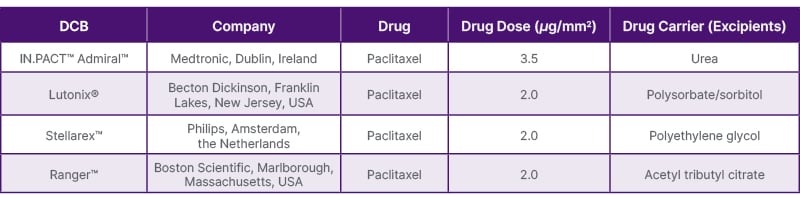
Table 1: Features of FDA-approved DCBs for peripheral above-the-knee interventions.
DCB: drug-coated balloon.
The citation source from Sato Y et al.27 was modified.
While different PCBs have been compared to each other, little published preclinical evidence exists after treatment with SCBs compared to PCBs. The authors first evaluated the vascular, myocardial, and pharmacokinetic effects of PCBs and SCBs in the hearts of swine after the treatment of coronary arteries with MT-SCB, SEL-SCB, Agent-PCB (Agent™ [Boston Scientific]), and POBA.10 In the histological assessment of coronary arteries, the arterial stenosis rate was comparable, and there were no late lumen enlargements in the Agent-PCB compared to the two types of SCB in 28 days. The Agent-PCB exhibited the highest score for SMC loss in the media among the four treatment groups, whereas endothelial cell loss, inflammatory score, and fibrosis were similar among the groups. This is shown in the representative histological images of the coronary arteries after treatment with the two types of SCBs, PCB, and POBA in Figure 2. These findings showed that sirolimus exhibits lower cytotoxicity compared to paclitaxel. However, the response of PCBs and SCBs in atherosclerotic lesions or neoatherosclerosis in DES or BMS restenosis is still relatively unproven.
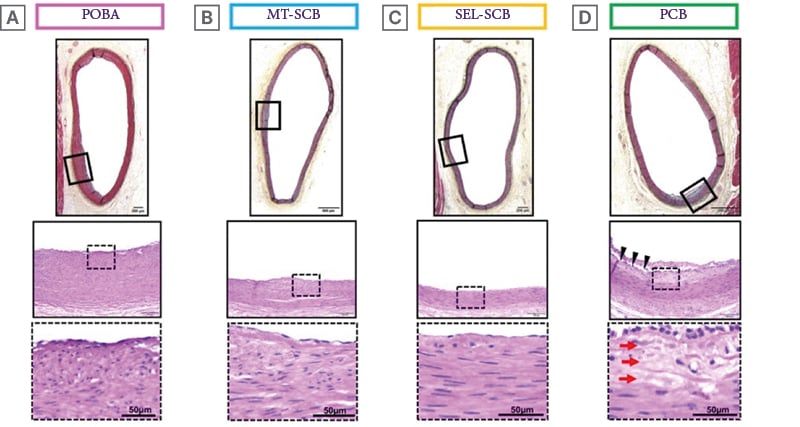
Figure 2: Representative histology of coronary arteries after treatment with different types of drug-coated balloons.
Histological images of coronary arteries treated with (A) POBA, (B) MagicTouch™-SCB (Concept Medical, Tampa, Florida, USA), (C) SELUTION SLR™-SCB (MedAlliance, Nyon, Switzerland), and (D) PCB. Boxed areas in upper images are shown magnified in the middle images. Dotted boxed areas in the middle images are shown as magnified images in the bottom row. All histology sections showed no stenosis with little intimal formation. The histology section from the coronary artery treated with PCB shows focal loss of SMCs in the media (red arrows), while there is no loss of SMCs in the media of the other groups. Arrowheads indicate cutting artifacts.
PCB: paclitaxel coated balloon; POBA: plain old balloon angioplasty; SCB: sirolimus-coated balloon;
SMC: smooth muscle cell.
The citation source is from Kawai K et al.10
The pharmacokinetic levels of the Agent-PCB in coronary arteries (648.3–3149 ng/g) at 28 days were similar to those previously reported in the lower extremity arteries (300–3000 ng/g).7,28 However, vascular tissue concentrations of SCBs (median in MT-SCB group: 21.3 ng/g; median in SEL-SCB group: 51.1 ng/g) were significantly lower than those for Agent-PCB at 28 days.10
Impact on Non-target Downstream Tissue Beds
The loss of drug into the body increases the potential for embolisation of the drug and excipients into downstream tissue. Some clinical cases have documented the occurrence of vasculitis after DCB treatment for peripheral artery disease.29,30 Whether this can be directly attributed to embolisation remains uncertain. Transient slow flow after PCB treatment31 and decreased coronary flow reserve are more commonly observed.32 The lack of approval of PCBs for below-the-knee interventions underscores the importance of this issue, with some analyses showing greater amputations in DCB groups.33 In preclinical studies, histological changes of downstream tissue following DCB treatment are predominantly identified as single or clustered multiple small vessels exhibiting varying degrees of fibrinoid necrosis, SMC apoptosis and loss, and adventitial inflammation or vasculitis, primarily composed of lymphocytes. How these findings translate to humans, where longer and larger DCBs are often used, remains uncertain.
In preclinical models, the overall percentage of distal emboli in skeletal muscle after DCB treatment for peripheral artery disease ranged from 25% to 42.9%.8 The downstream levels of paclitaxel concentration in skeletal muscle were comparative among the IN.PACT-PCB, Ranger-PCB, and Stellarex-PCB (216.5 versus 91.5 versus 101.9 ng/g, respectively).6,8 In the comparison of SCBs and PCBs in treated porcine coronary arteries,10 the frequency of identified downstream emboli was 36% for Agent-PCB, 15% for MT-SCB, and 25% for SEL-SCB. In the PCB group, 23% of histological sections presenting with emboli showed tissue injury (i.e., myocyte necrosis/scarring). There was no tissue injury in the MT-SCB and SEL-SCB groups. At 28 days post-treatment, median sirolimus concentration in the myocardium was 52.9 ng/g in the MT-SCB group, 0.0 ng/g in the SEL-SCB group, and 185.4 ng/g in the Agent-PCB group. An important factor when considering the impact of particulates generated by DCBs is their size. For the MT-SCB and SEL-SCB groups, particulate sizes ranged from 0.3 μm and 4 μm, respectively. Particulates from crystalline PCBs were 50 μm or greater.10,34 Thus, the size of the generated particulate is important in terms of effects on non-target organs. Although it is unknown how the degree of embolisation affects health conditions in the clinical setting, it is necessary to perform DCB treatments while acknowledging the preclinical data and tracking risks.
CLINICAL STUDIES
In-stent Restenosis
DCBs have been an established treatment option for ISR in most countries outside of the USA.35 However, the European Society of Cardiology (ESC)’s published 2024 guidelines36 have demonstrated a controversial shift, leading to a first-line recommendation for DESs over DCBs for ISR. The change in the newly published ESC guideline was driven in part by further negative data that showed higher rates of target vessel revascularisation (TVR) in patients treated with DCBs versus DESs for ISR when follow-up duration was extended beyond one year. The 36-month TLR and major adverse cardiac event (MACE) rates were significantly lower for PCBs compared with POBA in the PEPCAD-DES study.37 However, DCBs were as effective as DESs for the treatment of BMS-ISR, and less effective for DES-ISR when comparing rates of 3-year TLR in the other meta-analysis.38-40 The American College of Cardiology (ACC) and American Heart Association (AHA)41 do not have clear statements regarding the indication of DCB for ISR in the latest guidelines, likely because the first DCB (Agent) for ISR received approval just last year. Repeated stent implantation could have a significant negative impact on future treatment strategies, but this remains in the realm of theory. The future trends in DES-ISR treatment warrant close attention.
The first-in-man comparison of a novel SeQuent®-SCB (B. Braun, Melsungen, Germany [4 μg/mm2]) showed similar angiographic outcomes in the treatment of DES-ISR compared with a clinically proven SeQuent-PCB (SeQuent® Please NEO, B. Braun [3 μg/mm2]). After 6 months, in-segment late luminal loss was 0.21 ± 0.54 mm in the SeQuent-PCB versus 0.17 ± 0.55 mm in the SeQuent-SCB (p=0.794). Clinical events up to 12 months also did not differ between the groups.42 Furthermore, angioplasty with SCBs compared with DESs is associated with comparable rates of TLR, TVR, myocardial infarction (MI), and all-cause mortality at 2 years.43 Differences in effectiveness between DCBs and DESs for ISR may also be related to specific histopathologic and timing characteristics of neoatherosclerosis in the BMS- and DES-ISR subsets.44,45 In BMS-ISR, the neointimal tissue is composed of vascular SMCs and extracellular matrix with predominantly homogenous high-signal tissue echogenicity. In contrast, DES-ISR is more commonly associated with a layered pattern with heterogeneous tissue composition. This suggests that the preferred anti-restenotic and anti-inflammatory effectiveness of sirolimus compared to paclitaxel might be more advantageous for the treatment of DES-ISR. The MAGICAL ISR IDE study and SELUTION4ISR trial46 are ongoing studies that are investigating the effectiveness of SCB for ISR compared with the standard of care, such as DES and/or POBA.
Although the outcome of PCB treatment for BMS-ISR is promising, the use of DCBs for DES-ISR remains controversial. Accumulating clinical evidence that demonstrates the non-inferiority of SCB treatment compared to DES treatment for ISR, depending on the results, may help to establish the first-choice therapy for DES-ISR in the future.
De Novo Lesions – Small Vessel
Since stent implantation is challenging in SVD, with increased rates of restenosis compared to large vessel stenting, DCBs may have an advantage in the treatment of small vessels. Previous studies suggest that TLR rates and the risk of acute vessel occlusion were not significantly different between DCB and DES treatment at 12 months,47,48 supporting the use of DCBs in small vessel (<3.0 mm diameter) disease. In patients undergoing PCI for SVD, PCB angioplasty is associated with a reduction in MACE and a non-significant difference in target lesion failure (TLF) at 3-year follow-up compared with DES implantation.49,50 Furthermore, a meta-analysis of 20 studies comparing DCBs and DESs in patients with SVD demonstrated no significant difference in the risk of MACE, with incidence rates of 9.4% in the DCB group and 9.9% in the DES group.51 In acute coronary syndrome (ACS), a prespecified analysis of the BASKET-SMALL 2 trial demonstrated no interaction between the indication for PCI (ACS versus chronic coronary syndrome) and the treatment effect of PCBs versus DESs in patients with SVD.
The TRANSFORM I trial compared MT-SCBs to SeQuent-PCBs for the treatment of de novo SVD. The MT-SCB failed to achieve non-inferiority for angiographic net lumen gain at 6 months compared to the SeQuent-PCB, and less frequent late lumen enlargement (30.0% versus 53.7%; p=0.014) was observed with the SCB compared to the PCB.52 Paclitaxel, with high tissue permeability, likely facilitates late lumen enlargements caused by positive vascular remodeling. This effect may compensate for the lower acute gains in luminal dimensions that are initially observed with PCBs compared to DESs.53 Notably, clinical endpoints did not differ between the two devices in this trial; however, it is important to note that the TRANSFORM I trial did not include ACS with elevated cardiac biomarker values.
PCBs could be more effective than SCBs regarding SVD due to their greater facilitation of late lumen enlargement. However, there are still no long-term data after SCB treatment for de novo lesions, particularly regarding the efficacy in patients with ACS.
De Novo Lesions – Large Vessel
DCB angioplasty treatment of de novo lesions in large coronary vessels (>2.75 mm) remains controversial. A systematic review and meta-analysis of nine studies comprising patients with stable angina and ACS compared TLR between DCB and DES treatment. It showed that the incidence of TLR at a follow-up of 25.8±2.7 months was 4.3% versus 6.9%, and appeared to be similar between DCB and DES. Additionally, there were no differences in the incidence of cardiac death and myocardial infarction (MI).4 Meanwhile, the study from Gitto et al.,54 which is included in the meta-analysis,4 reported that the 2-year incidence of TLR and composite TLF were significantly higher in the DCB group compared to the DES group (TLR: 14.6% versus 3.5%; TLF: 18.2% versus 3.5%). However, the authors primarily attributed these findings to the greater lesion length in the DCB group, which reached up to 65 mm (median 40–82 mm), compared to 56 mm (median 46–66 mm) in the DES group. Regardless, these data suggest a higher risk of ISR in diffuse atherosclerosis and long lesions.4,54 In the DCB group, most TLR events occurred within the first 6 months and then remained stable, whereas in the DES group, the incidence of TLR gradually increased over time, likely reflecting the natural course of stent-related vascular remodelling. According to one analysis of 13,380 patients treated with second-generation DES, approximately 2% per year experienced very late ischaemic events, with an ischaemia-driven TLR incidence of 4.4% over a follow-up period of 1–5 years.3
In the REC-CAGEFREE I trial, the strategy of combining PCBs with rescue stenting did not achieve non-inferiority compared with intended DES implantation in terms of cardiovascular death, MI, and clinically and physiologically indicated TLR at 2 years.55 However, this study was designed to compare two devices: the Swide PCB (Shenqi Medical, Shanghai, China) and the Firebird 2 sirolimus-eluting stent (MicroPort, Shanghai, China), both of which are not widely used in Western countries. Furthermore, the appropriateness of DCB treatment following lesion preparation was defined as the absence of evidence of Type D, E, or F dissection. According to the international consensus group recommendations, any dissection classified as Type C or higher should be treated with stent implantation.56
Regarding the outcomes of DCBs for ACS, the REVELATION trial (including 120 patients) demonstrated that the DCB strategy for patients with ST-elevation MI was non-inferior to DES for those patients in terms of fractional flow reserve assessed at 9 months.57 The meta-analysis data consist of 13 studies, including the REVELATION trial, comparing DCBs and DESs for patients with acute MI on both de novo lesions and ISR. The analysis demonstrated that DCB was not inferior to DES treatment in terms of all-cause mortality (odds ratio [OR] 0.88; 95% CI: 0.43 to 1.8; p=0.73), cardiac mortality, (OR 0.59; 95% CI: 0.22 to 1.56; p=0.29), MI (OR 0.88; 95% CI: 0.34 to 2.29; p=0.79), stent thrombosis (OR 1.21; 95% CI: 0.35 to 4.23; p=0.76), TLR (OR 0.9; 95% CI: 0.43 to 1.93; p=0.8), and late luminal loss (mean difference –0.6; 95% CI: –0.3 to 0.19; p=0.64).58 This suggests that there is some, albeit limited, evidence for the use of PCBs in the treatment of ACS lesions.
Some other randomised controlled trials are ongoing, and their results will be crucial in better evaluating the performance of DCBs for large vessel de novo lesions in this clinical setting.23 The SELUTION DeNovo trial will compare a PCI strategy of SEL-SCB and provisional DESs to a PCI strategy of systematic DESs on target vessel failure at one and five years. Major exclusion criteria include lesions in the left main coronary artery, CTOs, ST-segment elevation MI, and non-ST-segment elevation MI. This is the largest randomised trial to date, and results are expected to be presented later this year.23 Regarding the efficacy of DCBs in CTO, the Co-CTO trial, which is the first randomised controlled trial to explore this topic, is an ongoing study investigating whether treatment with DCB is non-inferior to complete stenting of the CTO body.59
In DCBs for de novo large vessel disease, it may be essential to recognise the optimal lesion indications for DCB treatment, considering not only vessel size and ISR, but also plaque characteristics for the interventionalists. Further clinical trials evaluating PCB and SCB treatment for patients with CAD, especially ACS or CTO, are warranted in the future.
Bifurcation Lesions
PCI for bifurcation lesions is associated with a higher incidence of procedural complications and worse clinical outcomes, such as restenosis, compared to PCI for non-bifurcation lesions. A provisional one-stent strategy for bifurcation lesions decreased treatment time, contrast burden, and radiation exposure compared to a two-stent strategy.60 To address this, it has been recommended as the default strategy in ESC guidelines;61 however, it still results in relatively frequent restenosis, which may require revascularisation of the side branch. Recently, a randomised DCB-BIF trial of bifurcation lesions undergoing main vessel stenting with a severely compromised side branch showed that DCBs for side branch treatment resulted in a lower 1-year rate of the composite outcome compared with POBA treatment.62 Although the difference in this composite outcome was driven by spontaneous MI occurring more than 48 hours after the procedure, there were no significant differences between POBA and DCB groups in terms of success rate, all-cause mortality, clinically driven TVR, or stent thrombosis. Considering that spontaneous MI occurred early, whereas TLR developed relatively late, it is possible that most MIs were not eligible for revascularisation, and those that did not lead to revascularisation are of uncertain clinical significance.
Looking ahead, the ultimate goal of PCI for bifurcation lesions should be to avoid leaving any permanent metallic implants in the vessel, ideally achieving revascularisation with DCB treatment alone for both the main and side branches. The PEPCAD-BIF trial demonstrated that the restenosis rate was 6% at 9 months after PCB-alone, in which a PCB was used for both the main and side branches.63 Bruch et al.64 compared a PCB-alone treatment with a PCB treatment supplemented with additional BMS implantation as bailout for the main and/or side branches, reporting TLR and MACE in PCB-alone as 4.5% and 6.1%, respectively, at 9 months.64 The DEFINITION II trial compared a provisional one-stent strategy (using POBA for the side branch) with two-stent strategies such as the double kissing crush technique or culotte stenting techniques for complex bifurcation lesions. At one year, TLF occurred in 11.4 % using the one-stent strategy, and in 6.1% of patients using two-stent strategies.65 The authors summarise the recent evidence from the literature regarding the PCB and SCB in Table 2, and the ongoing important trial in Table 3.
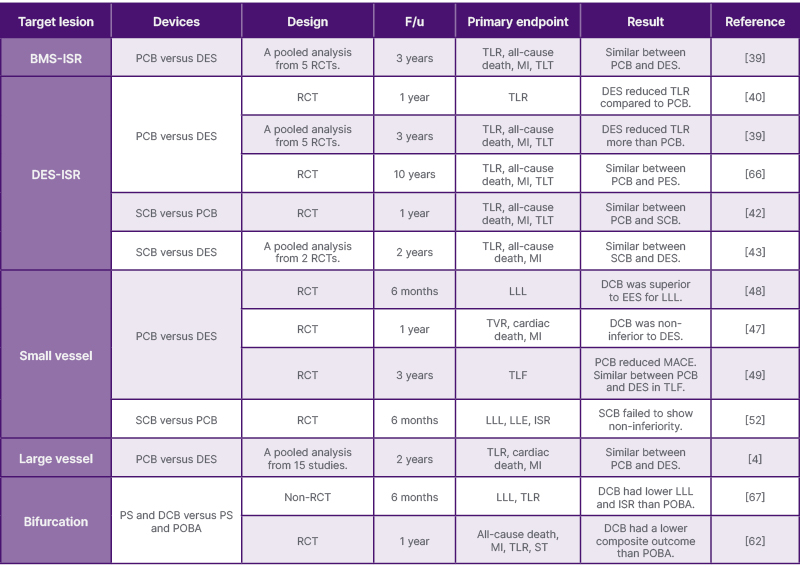
Table 2: The evidence for paclitaxel-coated balloons and sirolimus-coated balloons in percutaneous coronary interventions from representative literature.
BMS: bare-metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; EES: everolimus-eluting stent; F/u: follow-up duration; ISR: in-stent restenosis; LLE: late lumen enlargement; LLL: late luminal loss; MACE: major adverse cardiovascular events; MI: myocardial infarction; PCB: paclitaxel-coated balloon; PES: paclitaxel-eluting stent; POBA: plain old balloon angioplasty; PS: provisional stent; RCT: randomised controlled trial; SCB: sirolimus coated balloon; ST: stent thrombosis; TLF: target lumen failure; TLR: target lesion revascularization;
TLT: target lesion thrombus;
TVR: target vessel revascularisation.
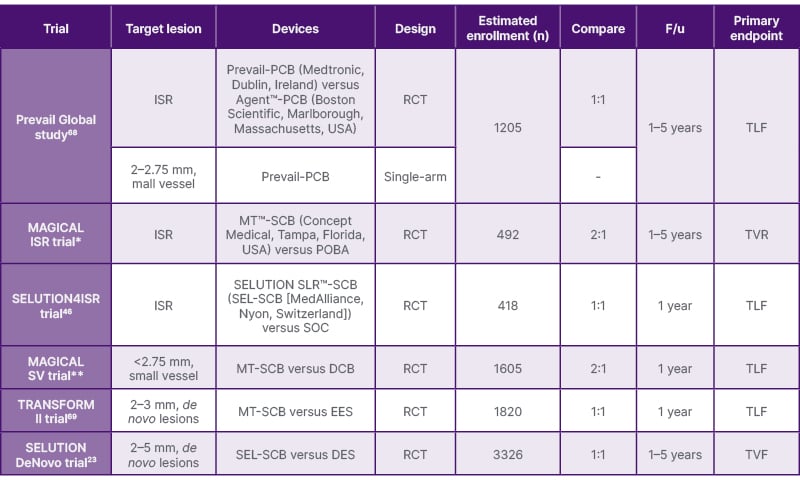
Table 3: Upcoming clinical trial.
DCB: drug-coated balloon; DES: drug-eluting stent; EES: everolimus-eluting stent; F/u: follow-up duration; ISR: in-stent restenosis; MT: MagicTouch; PCB: paclitaxel-coated balloon; POBA: plain old balloon angioplasty; RCT: randomised controlled trial; SCB: sirolimus-coated balloon; SOC: standard of care (DES and/or POBA); TLF: target lumen failure; TVR: target vessel revascularisation; TVF: target vessel failure.
Clinical trial ID: *NCT05908331; **NCT06271590.
DCB-alone treatment for the main and side branches is likely to show non-inferiority compared to the stent implantation treatment; however, direct comparative data between DCB-alone and DES are currently lacking. More data regarding the DCB-alone strategy are needed in the future.
NEWER DRUG-COATED BALLOONS
In clinical studies, only PCBs and SCBs have been investigated until recently; however, other types of innovative DCBs have evolved, such as the everolimus-coated balloon (ECB), the Biolimus A9™ (Biosensors International, Singapore), and a dual active pharmaceutical ingredient (API) DCB, which consists of both sirolimus and paclitaxel. In the preclinical data, ECBs with 2.5 μg/mm2 of drug per balloon surface showed low intimal area and intimal mean thickness, while ECBs with 7.5 μg/mm2 showed low stenosis compared to bare balloon and MT-SCB.70 The Chansu Vascular Technologies-ISR trial, which was a small clinical study, demonstrated the superior efficacy of the new ECB compared with POBA in the treatment of patients with ISR.71 A clinical trial evaluating the use of biodegradable-coated balloons in patients with CAD who require PCI for BMS- and DES-ISR will serve as a first-in-human experience.72 The dual API-DCB exhibited comparable inhibition of cell proliferation to the PCB, albeit at a markedly reduced total drug dose. The results of animal experiments demonstrated that the dual API-DCB is more effective in inhibiting intimal cell proliferation with insignificant downstream embolic effects and myocardial damage compared with the PCB. These findings indicate the potential for improved clinical outcomes and a greater safety profile than PCBs.73
CONCLUSION
In this review, the authors summarised the evolution of DCB technology based on preclinical data, as well as the vascular response, efficacy, and safety of DCBs. Furthermore, the authors examined the positioning of SCBs and PCBs within PCI strategies by incorporating clinical data. One of the major challenges of sirolimus is its lack of sustained tissue wall pharmacokinetics; however, advancements in DCB technology have addressed this issue, allowing sirolimus to become an integral component of DCB therapy. Compared to paclitaxel, sirolimus exhibits fewer late lumen enlargements, but it is considered to have superior anti-restenotic and anti-inflammatory properties.
The characteristics of PCBs may explain their well-established efficacy in the treatment of SVD. However, clinical data supporting which lesions sirolimus may potentially treat are currently lacking, and no definitive conclusions can yet be drawn. Moreover, each DCB formulation (whether PCB or SCB) needs to be tested for specific clinical indications before its efficacy and safety can be established. There should be no class effect given the enormous differences in how DCBs are formulated. For now, DCB-only strategies should be considered for selected lesions where outcomes are anticipated to be non-inferior to DESs, taking into account patient and lesion characteristics, the quality of lesion preparation, presence or absence of thrombus, bleeding risk, and other procedural factors.
Ongoing research is exploring the use of other drugs, such as everolimus, in DCB therapy. The impact of these next-generation devices remains to be elucidated in future investigations. The treatment strategies in PCI may need to be tailored to the characteristics of the lesion and the speed of cell proliferation, similar to cancer treatment. Regardless, DCBs are here to stay, and as data evolve, their exact role in coronary intervention will continue to develop, as will the technology itself. Ultimately, all of this will drive the next wave of innovation in interventional cardiology.