Abstract
Background: While transcatheter aortic valve implantation (TAVI) is a well-established therapy for elderly patients with aortic stenosis, its role in treating pure non-calcified aortic regurgitation (AR) remains limited due to anatomical challenges, device limitations, and off-label use.
Methods: This review examines current evidence from registries and meta-analyses focusing on TAVI outcomes in patients with pure native AR, highlighting technical considerations, patient selection criteria, and device performance.
Results: TAVI in AR presents unique procedural challenges due to the lack of annular and leaflet calcification, frequent aortic root dilation, large and elliptical annuli, and high stroke volumes. These features compromise prosthesis anchoring and fluoroscopic visualisation, increasing the risk of prosthesis migration, paravalvular leak, and procedural failure. Both off-label transcatheter heart valves, originally designed for aortic stenosis, and dedicated on-label devices have been used, each with specific advantages and limitations. However, the most critical determinant of procedural success is not the type of device per se, but rather a thorough understanding of the anatomical challenges inherent to AR. Accurate preprocedural imaging, appropriate oversizing strategies, and tailored implantation techniques are essential to achieve optimal outcomes.
Conclusions: TAVI is an emerging option for selected patients with pure AR, using either off-label or on-label devices. Success depends primarily on recognising and addressing the anatomical and technical complexities that differentiate AR from aortic valve stenosis, underscoring the importance of individualised patient assessment and procedural planning.
Key points
1. Transcatheter aortic valve implantation (TAVI) is a well-established treatment for elderly patients with aortic stenosis but remains off-label in pure non-calcified aortic regurgitation (AR). As there is a significant number of patients with AR that are high-risk or inoperable referred for treatment, there is an emergent clinical need for less invasive alternatives.2. This review provides a comprehensive overview of the current evidence and technical considerations surrounding TAVI in pure AR. It highlights anatomical challenges unique to AR, compares off-label and dedicated devices, and discusses procedural planning and patient selection strategies to optimise outcomes.
3. The success of TAVI in AR depends on both the prosthesis type and understanding the anatomical differences from aortic stenosis. Careful preprocedural imaging, individualised oversizing, and recognition of anchoring difficulties are crucial. Both, off-label and on-label transcatheter heart valves can be used effectively in appropriately selected patients that are high-risk. On-label devices are associated with a higher technical success but to date, they’re not extensively available on the market.
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) is now an established treatment option for elderly patients with severe aortic valve stenosis (AS). On the other hand, few data are available on TAVI in patients with aortic regurgitation (AR). 1-3 The incidence of AR increases with age, affecting nearly 5% of individuals aged ≥75 years.4 It is not uncommon for these patients to be at high surgical risk, particularly due to advanced age and comorbidities. Once patients with AR become symptomatic, the mortality rate among those who do not undergo surgical intervention reaches 20% per year.5 Furthermore, according to data from The Euro Heart Survey on valvular heart disease, only 5% of these patients receive surgical aortic valve replacement.6 It is therefore evident that there is a pressing need for a less invasive approach to treat this patient population. Despite the widespread adoption of TAVI for AS, its use in AR remains limited. This discrepancy is mainly related to the anatomical features of AR (few or no calcium on the aortic valve leaflets, no fluoroscopic markers, and an enlarged aortic root/left ventricular outflow tract). As a result, the transcatheter heart valves (THV) may be prone to malpositioning, migration, embolisation, and incomplete annular sealing, leading to significant residual paravalvular leak (PVL).7 The available data on TAVI in pure AR are mostly deriving from the off-label use of various THVs.8,9 More recently, dedicated THVs have shown improved outcomes compared to off-label devices.10,11 However, these dedicated THVs still have some limitations in terms of sizes and unavailability in everyday clinical practice.
The aim of this review is to summarise the existing data and evidence on TAVI as a treatment option for patients with AR who are high-risk/inoperable. Furthermore, specific technical aspects on TAVI in AR will be discussed.
PATIENT SELECTION AND INDICATIONS TO TREATMENT
According to the 2022 European Society of Cardiology /European Association for Cardio-Thoracic Surgery guidelines, patient selection for intervention in severe chronic AR is primarily guided by the presence of symptoms, left ventricular (LV) function and dimensions, and associated aortic root dilation. In patients who are symptomatic, intervention is strongly recommended regardless of LV ejection fraction (LVEF), provided that the surgical risk is not prohibitive. In patients who are asymptomatic, the decision to intervene is influenced by LV dysfunction (LVEF ≤50%) or significant LV dilation (LV end-systolic diameter; LVESD >50 mm), as these parameters are associated with adverse outcomes (Class I, level of evidence B).12 Recent evidence suggests that indexing LVESD to body surface area may refine patient selection, with proposed cut-offs of 20–22 mm/m², although these criteria currently support a Class IIb recommendation.13,14 In such cases, intervention may be considered (Class IIb, level of evidence C) In addition, progressive LV dilation or a gradual decline in LVEF, particularly in patients with significant LV enlargement (LV end-diastolic diameter; LVEDD >65 mm), may also prompt consideration of intervention, even in the absence of symptoms. Comprehensive evaluation by a multidisciplinary heart team is emphasised, accounting for age, comorbidities, and overall risk profile.12 For patients who are not suitable candidates for surgical aortic valve replacement ,TAVI may be considered at selected centres. In this context, TAVI is typically reserved for patients at high or prohibitive surgical risk, with careful consideration of anatomical and procedural feasibility. Overall, patient selection for TAVI in severe AR relies on an integrated approach, balancing the severity of regurgitation, LV remodeling and dysfunction, symptom burden, and operative risk, with final decisions supported by a heart team-based consensus.12,15
TECHNICAL AND ANATOMICAL CHALLENGES
TAVI was designed and validated as a treatment for severe AS. Based on the different anatomical scenario, this approach cannot be immediately applied to pure non-calcified AR. AS is often characterised by extensive calcifications at both the annulus and leaflet levels, which facilitate the anchoring of both self-expanding (SE) and balloon-expandable (BE) THVs, as well as provide a fluoroscopic landmark for guiding the implant. Patients with pure non-calcified AR often exhibit little to no calcium, elliptical and large annulus, dilation of the aortic root and ascending aorta, and a large stroke volume with turbulent regurgitant jet. This may also be associated with a bicuspid aortic valve anatomy. These characteristics represent a challenge in the transcatheter treatment of this valvular heart disease. The main issue when dealing with pure non-calcified AR is anchoring the THV to the annulus. Due to the lack of calcium, a significant THV oversizing (15–25%) and a deeper landing into the left ventricular outflow tract (particularly in ‘tubular’ and ‘flared’ anatomies) are necessary to secure the THV and reduce the risk of migration, embolisation, and/or significant AR. As a result, the required THV size is often beyond the range of most commonly available devices. The need for substantial oversizing is also theoretically associated with a higher risk of advanced conduction disturbances, potentially necessitating permanent pacemaker implantation. Furthermore, the regurgitant volume can complicate the identification of correct implantation views, especially in cases of leaflet prolapse. All these technical challenges may explain the higher rates of surgical crossover and peri-procedural mortality compared to AS cases.16-18
TRANSCATHETER HEART VALVES IN PURE NON-CALCIFIED AORTIC REGURGITATION
TAVI for pure non-calcified AR has evolved significantly over the past decade. THVs currently used in this context are broadly categorised into: off-label, originally developed for AS but repurposed in selected AR cases; and on-label, which are specifically designed and approved for AR.19 Figure 1 provides an overview of THVs used in patients with pure native AR, highlighting off-label and on-label THVs, categorised into SE and BE devices. It includes sizing ranges in terms of diameters, perimeters, and annulus area. Although the clinical application of TAVI in AR is expanding, available data remain limited compared to AS, with most evidence derived from retrospective registries or small prospective studies.
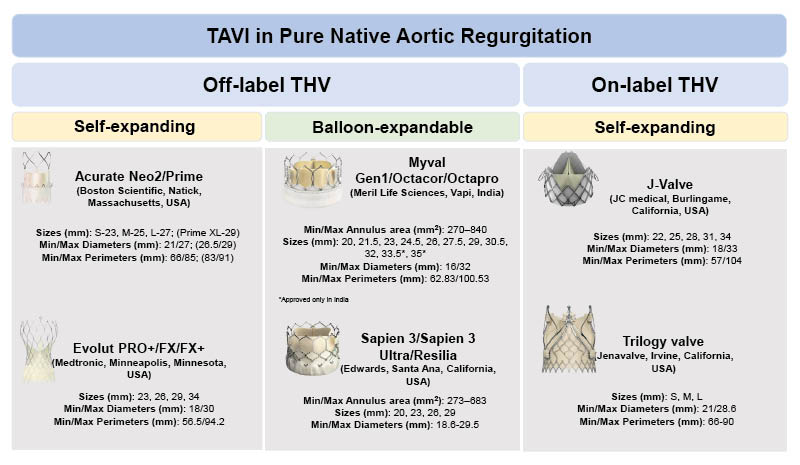
Figure 1: Transcatheter heart valves types used in patients with pure non-calcified aortic regurgitation.
TAVI: Transcatheter aortic valve implantation; TAVR: transcatheter aortic valve regurgitation; THV: transcatheter heart valve
Off-Label THVs in Pure Aortic Regurgitation
The CoreValve™ (Medtronic, Minneapolis, Minnesota, USA) was the most widely first-generation SE, supra-annular, non-recapturable/retrievable THV used for the treatment of pure native AR. The nitinol structure of the frames provided an acceptable stability during implantation, even in the absence of calcification. Moreover, this THV was believed to allow for a considerable degree of oversizing (3 sizes available: 23 mm, 26 mm, and 29 mm; covering perimeters up to 84.8 mm) with a low-risk of annular rupture. However, CoreValve was associated with a significant rate of second THV deployment and more than moderate residual AR due to an incomplete annular sealing.17 The introduction of the SE supra-annular THVs Evolut R, and Evolut Pro/Pro+ (Medtronic, Minneapolis, Minnesota, USA)20 not only enabled the use of a recapturable and repositionable bioprosthesis, but also offered a wider option of sizes (23 mm, 26 mm, 29 mm, and 31/34 mm) to accommodate larger annular perimeters. The external cuff on the Pro/Pro+ models helped minimise PVL.17,21 Indeed, the most commonly Evolut implanted size in AR cases was the 34 mm.22 Early experiences with first-generation SE THVs reported procedural success rates ranging from 74% to 100%, with frequent complications such as moderate-to-severe post-procedural AR (9%) and second THV implantation (7%). 23,24
Another SE supra-annular THV used off-label for pure AR is the ACURATETM family (Boston Scientific, Natick, Massachusetts, USA). Its main limitation is the limited number of sizes available (23 mm, 25 mm, and 27 mm) provided by the ACURATE neo and neo 2 models (the latter featuring a taller external skirt). This restricts treatment to ‘small annuli’ only. Studies on the ACURATE neo THV for pure native AR showed device success rates ranging from 87.5% to 100%, influenced by oversizing and implant height. Oversizing ≥10% (commonly using the 27 mm ‘L’ size for the ACURATE) improved outcomes but was associated with increased pacemaker implantation rates. The newer ACURATE Prime XL (29 mm) offers broader anatomical compatibility, although no data are currently available for its use in either AS or AR.24-27
Early evidence on a BE THV performance for treating pure native AR emerged in 2016. Urena et al.28 reported successful outcomes in three inoperable patients treated with the SAPIEN 3 THV (Edwards Lifesciences, Irvine, California, USA), emphasising the importance of oversizing (ranging from 16% to 27%) to ensure THV stability and prevent displacement, with all patients demonstrating improved New York Heart Association class and no residual AR.28 More extensive data come from the multicenter French S3AR study (2015–2021), involving 49 patients, which reported a procedural success rate of 94.6%. Four cases of THV embolisation occurred, all associated with <15% oversizing. These findings reinforced the recommendation for at least 15% oversizing and lower implantation depth, which may also explain the 35% permanent pacemaker rate. Notably, 70% of the enrolled patients received a 29 mm SAPIEN THV, implantable in annular areas up to 683 mm², while the largest annular area among the enrolled patients was 605 mm².29 Since 2019 when received CE mark, the Myval BE THV (Meril Life Sciences, Vapi, India) expanded annular sizing up to 840 mm² (diameter of 32.7 mm), addressing the anatomical demands of AR.30,31 In the study by Sanchez-Luna et al.32 113 patients, treated with this novel BE THV, achieved a 94.7% technical success rate, with oversizing averaging 17.9%. Moderate or greater residual AR occurred in 8.9% of cases, while THV embolisation (3.5%) was associated with unfavourable left ventricular outflow tract (LVOT) morphology, specifically a tapered anatomy where the LVOT is larger than the annulus.32
The technical (e.g., operator skills) and technological (e.g., THV repositionability/retrievability and external sealing skirt) improvements over the past decade have been associated with higher success rates (61.3–81.1%; p<0.001), a reduced need for implantation of a second THV (12.7% versus 24.4%; p=0.007), a lower incidence of at least moderate residual AR (4.2% versus 18.8%; p<0.001), and decreased 1-year cardiovascular mortality (9.6% versus 23.6%; p=0.008)21,22 following the off-label use of THVs in pure AR.19,21
Similarly, Sawaya et al.33 found Valve Academic Research Consortium-2 device success improved from 54% to 85% (p=0.01), and 30-day clinical efficacy increased from 46% to 75% with newer generation THVs (p=0.01).33
Among the studies evaluating the performance of first versus novel generation off-label THVs in pure native AR, Yoon et al.,18 FRANCE TAVI,19 and the PANTHEON34 studies are the most representative. The FRANCE TAVI, and PANTHEON studies showed technical success of 85.5% and 83.6% respectively, according to Valve Academic Research Consortium (VARC)-3 criteria. While both studies identified the need for a second THV implantation and THV embolization/migration as the main complications, FRANCE TAVI (which enrolled the older population) reported a higher permanent pacemaker implantation (36% versus 22%) and a worse long-term mortality (53.5% at 4 years). In contrast, PANTHEON had a lower (17.1%) 1-year composite endpoint (all-cause mortality and heart failure rehospitalisation) and emphasised the prognostic impact of THV embolisation or migration. It also provided a detailed comparison of BE versus SE THVs, finding similar efficacy but differences in anatomical suitability and procedural characteristics. FRANCE TAVI highlighted oversizing as beneficial yet risky, while PANTHEON identified post-dilation as a predictor of THV migration. Both studies stressed the need for dedicated THVs to overcome the unique anatomical challenges of pure non-calcified AR.19,34 Table 1 summarises the most relevant data on outcomes, technical and device success, as well as complication rates reported above.
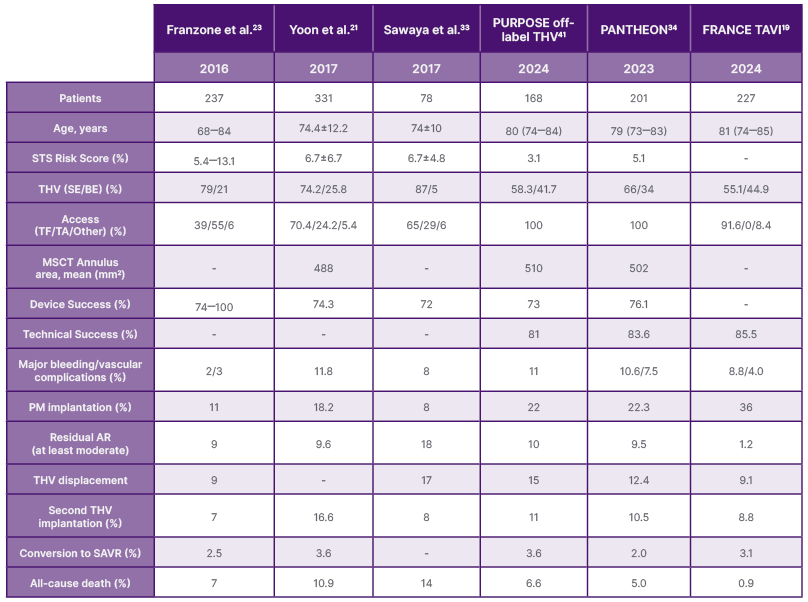
Table 1: Main characteristics and outcome of transcatheter heart valve used off-label in aortic regurgitation.
AR: aortic regurgitation; BE: balloon-expandable; ME: mechanically expanded; MSCT: multisclice computed tomography; PM: pacemaker; SAVR: surgical aortic valve replacement; SE: self-expanding; STS: Society of Thoracic Surgeons; TA: transapical; TF: transfemoral; THV: transcatheter heart valve.
On-label THVs in Pure Aortic Regurgitation
One of the main limitations of first and newer generation THVs used off-label was the lack of anchoring structures to secure the prosthesis to the annulus in the absence of calcium on the leaflets. To overcome this issue, specifically designed THVs have been developed to be used in patients suffering from severe pure non-calcified AR. The two main AR dedicated THVs are the JenaValve Trilogy™ (JVT) system (JenaValve Technology, Irvine, California, USA) and the J-Valve™ system (JC Medical, Burlingame, California, USA), both featuring dedicated anchoring mechanisms to compensate for the lack of valvular calcification. Table 2 summarises the most relevant evidence, showing how these devices have demonstrated promising outcomes with high procedural success rates and reduced complications, particularly regarding PVL.10,11 JVT is the only CE-marked THV approved specifically for AR. It is a SE prosthesis delivered via an 18 Fr transfemoral system, with a nitinol frame supporting a porcine pericardial valve. This THV is available in three sizes (23 mm, 25 mm, 27 mm). Its key innovation lies in the integrated ‘feelers’ that align the THV with the native cusps, and enable anchoring by clamping the cusps between the frame and locator elements. This design enables secure fixation without relying on calcification or other anatomical landmarks.35 Initial outcomes for the JVT came from a German registry involving nine patients treated via transapical access, reporting a 97% procedural success rate, and 30-day and 6-month mortality rates of 13% and 19%, respectively.36 These findings were later confirmed in additional transapical studies.37,38 In 2023, Adam et al.39 reported outcomes from 58 patients who underwent transfemoral JVT implantation, achieving 100% technical success and 98% device success at 30 days, with no cases of moderate or severe PVL.39 The ALIGN-AR study further validated these results in 180 patients (mean age 75.5 years), showing 95% technical success, 30-day mortality of 2%, and significant improvement in New York Heart Association class and left ventricular mass at 1-year.40 The potential benefits associated with the on-label use of a dedicated THVs with AR were explored in the PURPOSE study. JVT was compared with off-label THVs in 256 patients with inoperable AR. JVT showed superior VARC-3 technical success (98% versus 81%; p<0.001), and device success (95% versus 73%; p< 0.001) compared with off-label THVs. JVT significantly reduced complications such as THV embolisation (1.1% versus 15%; p<0.001) and moderate or greater residual AR (1.1% versus 11%; p=0.007). However, 1-year clinical outcomes, including mortality and heart failure rehospitalisation, were similar between groups (17.2% versus 14.4%).41
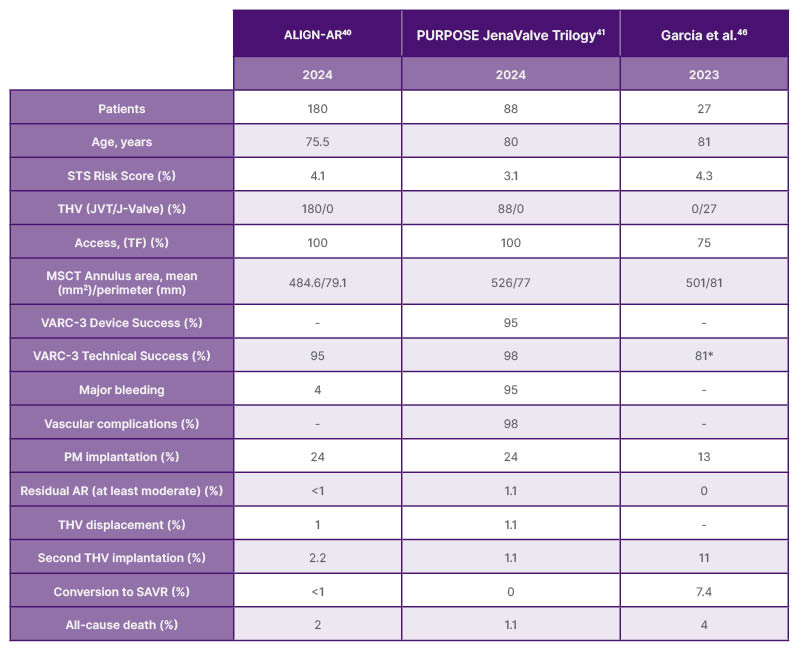
Table 2: Main characteristics and outcome of trans-catheter heart valve used on-label in aortic regurgitation.
*Procedural success
AR: aortic regurgitation; JVT: JenaValve Trilogy; MSCT: multislice computed tomography; PM: pacemaker; SAVR: surgical aortic valve replacement; SE: self-expanding; STS: Society of Thoracic Surgeons; TF: transfemoral; THV: transcatheter heart valve; VARC: Valve Academic Research Consortium.
The J-Valve shares technical and functional characteristics with the JenaValve. Specifically, the J-Valve also aligns with the native aortic valve cusps, followed by a grasping phase facilitated by protrusions in the THV structure. The anchoring phase then involves the release of the bioprosthesis via a self-expanding system. The J-valve consists of two main components: the valve-locating feature, composed of three U-shaped anchor rings designed to lodge in the sinuses of Valsalva, and a self-expanding nitinol frame with bovine pericardium leaflets and a polyester skirt covering the entire outer surface of the valve.17 As with the Jena, the first J-Valve implants were performed via the transapical route, with the first transfemoral (18 to 21-F) cases conducted only in 2019.42–44 More recent data come from authors including Liu et al.45 and, more recently, Garcia et al.46 who reported outcomes of J-Valve use for treating pure native AR in 2018 and 2023, respectively, both showing positive procedural success rates (97.7% and 81%, respectively). An interesting finding reported by Garcia et al.46 concerns the approach, which was transfemoral in 75% of cases, and the perimeter, which exceeded 85 mm in 38% of cases, a parameter that represented an exclusion criterion in previous studies with the JVT.
A recent meta-analysis by Samimi et al.47 encompassing 2,162 patients with high-risk AR (mean STS score 5.6 ± 0.1%) reported lower 30-day (3% versus 9%) and 1-year (6% versus 24%) mortality in patients treated with dedicated THVs (n=588 patients with JVT, and n=605 with J-Valve) versus off-label THVs. Device success was higher (93% versus 82%), with fewer complications, including residual AR (2% versus 5%), valve embolisation (2% versus 8%), and pacemaker implantation (11% versus 20%).47 Another meta-analysis of 1,851 patients echoed these findings and provided deeper insights into anatomical considerations, access routes, and anchoring mechanisms, emphasising higher device success according to VARC-3 (97.8% versus 89.9%), lower 30-day mortality (2.6% versus 5.1%), and fewer complications including PVL and pacemaker implantation with on-label versus off-label THVs.7 Both studies confirm the superiority of on-label, new-generation THVs in managing patients with severe AR in high-risk, particularly within the early post-procedural window.7 However, the main current limitation of dedicated THVs for AR is their size range (JVT covers perimeters from 66–90 mm, while the J-Valve ranges from 57–104 mm), which does not allow for sufficient oversizing in large and extra-large native annuli. Furthermore, both THVs are yet not available for routine clinical use.
CLINICAL AND TECHNICAL IMPLICATIONS
The data presented thus far highlight the numerous technical challenges associated with TAVI in pure native AR, the potential complications, and the procedural characteristics that may be linked to unfavourable technical or clinical outcomes. When performing preprocedural planning, it is essential to understand the anatomical characteristics of each individual patient in order to anticipate potential technical limitations of the available devices and select the THV best suited to the specific case. Key factors to always consider include: aortic valve morphology (bicuspid versus tricuspid), annular (perimeter and area) and calcific burden (leaflets), the underlying mechanism of AR (cusp prolapse or retraction versus coaptation defect due to ascending aorta dilation), left ventricular size and function including measurements of the left ventricular outflow tract, and finally the dimensions and of the aortic root and ascending aorta. Regarding prosthesis sizing, selection should be based on either the annular perimeter or area, depending on whether a BE or SE THV is used. In most cases of pure native AR, a significant degree of oversizing is recommended (15% to 25%). One commonly described method for calculating the degree of oversizing involves comparing the THV diameter to that of the annulus in order to derive the prosthesis-to-annulus coverage index.22,48 An appropriate degree of oversizing is crucial to achieve sufficient anchoring and optimal annular sealing, both of which are necessary to ensure favourable procedural outcomes.49 Another challenge to consider is the need for device stability. The lack of fluoroscopic markers due to absent annular calcification can complicate the procedure. In this context, it is important to underline that SE bioprostheses offer multiple anchoring zones: one in the ascending aorta; one at the level of the cusps; and another at the annulus. On the other hand, actually available SE THVs do not provide enough sizes to allow an adequate oversizing, particularly in large annuli. BE THVs instead, and Myval in particular, cover larger areas. In some cases, the implantation views are not easy to be found because of the regurgitant volume. Positioning two pigtail catheters, one in the left coronary cusp and the other in the non-coronary cusp, or placing three fluoroscopic markers (e.g., a pigtail catheter and two J-starter 0.035 inches wires), can provide valuable anatomical reference points, thereby reducing both the number of aortograms required and the total contrast medium dose.5 Furthermore, TAVI procedures in AR often require prolonged ventricular pacing, typically ‘fast’ pacing up to 140 bpm during SE valve deployment and ‘rapid’ pacing up to 220 bpm during BE implantation, to reduce systolic arterial pressure and regurgitant volume, thereby enhancing THV stability during deployment.50
In cases of at least moderate residual AR, it is important to note that post-dilation is associated with a higher risk of device embolisation. In such scenarios, implantation of a second THV may be considered.22
A structured algorithm considering valve morphology, annular dimensions, calcific burden, and ventricular function enables appropriate device selection and sizing. Oversizing strategies and deployment techniques must be tailored to anatomical challenges, especially in the absence of calcification. The use of SE- versus BE THVs depends on anchoring requirements and annulus size. Ultimately, the clinical and economic viability of TAVI in AR hinges on optimising patient-device matching through precise preprocedural assessment. Figure 2 presents a detailed case of a BE THV used off-label for treating AR, illustrating pre-procedural anatomical assessment via MSCT, challenges posed by non-calcified leaflets, and significant aortic angulation. Hemodynamic and aortographic images highlight baseline and final outcomes, while fluoroscopic markers guide optimal valve positioning.
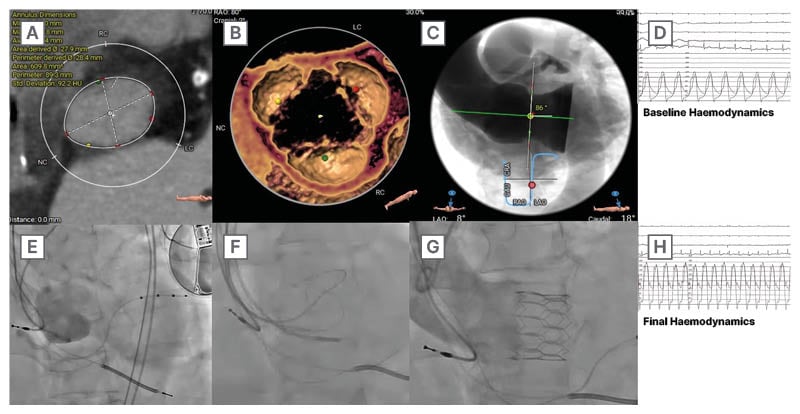
Figure 2: Case example of a balloon-expandable transcatheter heart valve used off-label for the treatment of aortic regurgitation.
TAVR: transcatheter aortic valve regurgitation; THV: transcatheter heart valve.
While TAVI has demonstrated cost-effectiveness in AS, its application in pure native AR is complicated by higher procedural costs, increased use of multiple valves per case, and higher rates of valve malpositioning or embolisation, which elevate the overall economic burden. Furthermore, pure native AR patients often require on-label THVs, which are more expensive, especially if compared to off-label devices. A recent cost-utility analysis reported that, under current clinical conditions, TAVI for pure native AR may exceed commonly accepted willingness-to-pay thresholds in both U.S. and European models. Additionally, the absence of long-term durability data in this population undermines assumptions used in economic modelling. Although early clinical outcomes are favourable, from a health economics perspective, TAVI in this population is still currently considered cost-effective when compared to SAVR.51,52
CONCLUSION
Pure non-calcified AR represents a complex clinical and anatomical entity, for which percutaneous treatment could be considered as an alternative to the traditional surgical approach in selected patients, particularly when cardiac surgery entails an excessively high risk or when conservative management may be associated with a poor prognosis. Although dedicated THVs for AR have been associated with better performance as compared to devices used off-label, they are associated with size limitations and poor availability in everyday clinical practice. Based on this, current-generation (off-label, non-dedicated) THVs are the only alternative for AR patients during this time period.
Meticulous preprocedural planning, based on multimodality imaging, is essential to ensure accurate patient selection and the identification of the most appropriate THV for the specific anatomical context.