Abstract
Stent fractures are rare but significant complications following percutaneous coronary intervention (PCI), particularly in the treatment of chronic total occlusions using advanced techniques like the reverse-controlled antegrade and retrograde tracking method. This case is unique due to the occurrence of a rare Type IV stent fracture after such a procedure, which adds valuable insights to the scientific literature on prevention and management strategies for this complication.
A 59-year-old male with hypertension and ischaemic heart disease presented with exertional dyspnoea. Coronary angiography revealed a chronic total occlusion of the right coronary artery. He underwent successful PCI using the reverse controlled antegrade and retrograde tracking technique, which involved the placement of five overlapping drug-eluting stents. At 2 months post-procedure, follow-up angiography detected a rare Type IV stent fracture in the mid-right coronary artery. A repeat PCI was performed to bridge the fractured stent segments, restoring vessel patency. The patient was discharged on optimised medical therapy, advised on lifestyle modifications, and scheduled for regular follow-up to monitor his cardiovascular health.
This case highlights the critical importance of meticulous procedural planning and consideration of vessel dynamics in complex PCI procedures to prevent stent fractures. Early detection through vigilant post-procedural monitoring enabled prompt management of the complication. The main takeaway is that careful stent deployment strategies and diligent follow-up are essential for improving patient outcomes in interventional cardiology.
Key points
1. Stent fractures, though uncommon, pose significant clinical risks, such as in-stent restenosis and thrombosis. This is especially true in complex percutaneous coronary intervention cases involving long, overlapping drug-eluting stents in mobile vessels like the right coronary artery.2. This article presents the unique case of a Type IV stent fracture. It was incidentally detected 2 months after reverse-controlled antegrade and retrograde tracking-guided percutaneous coronary intervention for a chronic total occlusion of the right coronary artery. It also details the combined use of an additional drug-eluting stent and a covered stent to restore vessel integrity, alongside a comprehensive literature review of risk factors, diagnostic modalities, and management strategies.
3. Healthcare professionals should prioritise meticulous stent selection and deployment techniques, employ routine post-procedural imaging (e.g., StentBoost [Philips, Amsterdam, the Netherlands], intravascular ultrasound, optical coherence tomography) for early fracture detection, and maintain vigilant follow-up, especially in chronic total occlusions and overlapping-stent scenarios, to optimise patient outcomes.
INTRODUCTION
This case is unique because it documents a rare occurrence of a Type IV stent fracture following percutaneous coronary intervention (PCI), which was performed for a chronic total occlusion (CTO) of the right coronary artery (RCA) using the reverse controlled antegrade and retrograde tracking (CART) technique. The fracture was discovered incidentally during routine angiographic follow-up in a 59-year-old male patient who was asymptomatic, highlighting the importance of vigilant post-procedural monitoring even when patients are symptom-free. The stent fracture occurred at the site of a Xience Skypoint™ (Abbott, Abbott Park, Illinois, USA) stent within a series of five overlapping drug-eluting stents (DES), which is a complex stenting strategy that is seldom reported in the literature.
Furthermore, the management of this case involved an innovative approach: deploying an additional DES to bridge the fractured segments, followed by the placement of a covered stent due to concerns about potential aneurysm formation or coronary perforation. The combination of a rare complication, an asymptomatic presentation, and a unique intervention strategy enhances our understanding of stent fractures. It underscores the need for meticulous procedural planning, consideration of vessel dynamics, and the use of advanced imaging techniques for early detection and effective management of such complications in interventional cardiology.
Patient Information
A 59-year-old male patient, who smokes and has a history of hypertension and ischaemic heart disease, previously underwent stenting of the left anterior descending artery. He arrived at the cardiology clinic with exertional dyspnoea, although both the ECG and resting echocardiogram results were normal. Following the acquisition of informed consent, the patient underwent PCI to the RCA, during which five overlapping DESs were placed in the CTO of the RCA. Utilising the reverse CART approach, they were positioned from distal to proximal as follows: Orsiro® Mission (BIOTRONIK, Berlin, Germany) 2.25×26 mm, Onyx Frontier™ (Medtronic, Dublin, Ireland) 2.5×38 mm, Resolute Onyx™ (Medtronic) 3.0×30 mm, Xience Skypoint 3.0×28 mm, and Synergy Megatron™ (Boston Scientific, Marlborough, Massachusetts, USA) 3.5×8 mm. This mix of stents was due to the availability and sizing, and at the end of the procedure, the StentBoost (Philips, Amsterdam, the Netherlands) was used to ensure optimal opposition. The patient was scheduled for elective admission to undergo follow-up coronary angiography after two months. After giving informed consent, the patient underwent coronary angiography via the right radial artery using a diagnostic 5F Judkins Left (JL) 3.5 catheter, which revealed a non-obstructed left coronary system. Upon visualising the RCA with a diagnostic 6F Judkins Right (JR) 4.0 catheter, a Type IV stent fracture was revealed at the mid-segment where the Xience Skypoint stent was located. The diagnosis was made with StentBoost and confirmed by intravascular ultrasound (IVUS) (Figure 1).
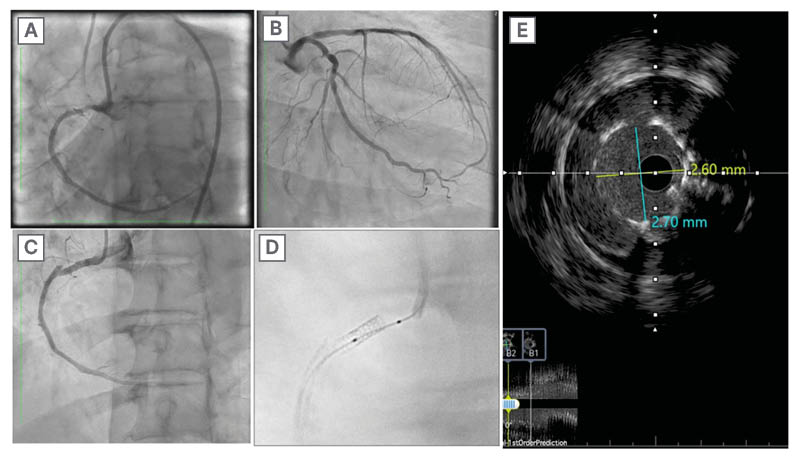
Figure 1: Multimodality imaging of a mid-right coronary artery Type IV stent fracture after primary stenting.
A) Final result of the first procedure (after stent implantations in RCA). B) coronary angiography of the left system showing no obstructions. C) RCA showing a Type IV stent fracture at the mid-segment. D) StentBoost (Philips, Amsterdam, the Netherlands) to fractured stent. E) IVUS showing minimal stent area of 7.02 mm².
IVUS: intravascular ultrasound; RCA: right coronary artery.
TIMELINE
Stent fracture (SF) is an uncommon complication of PCI that can lead to adverse clinical outcomes such as in-stent restenosis (ISR), thrombosis, and acute coronary syndromes.1-3 The introduction of coronary stents revolutionised the treatment of coronary artery disease by providing a scaffold that prevents vessel closure post-angioplasty.3 However, the mechanical integrity of these stents is crucial for their long-term efficacy.
SF refers to a partial or complete separation of the stent struts, which can compromise the vessel’s patency and lead to significant clinical implications.4,5 A recent meta-analysis reported that the type of stent, implantation technique, and patient-specific factors influence the incidence of coronary stent fractures, which varies widely in the literature between 4.8–5.5%.6
Coronary stents are produced using diverse platforms that considerably influence their mechanical characteristics, biocompatibility, and long-term efficacy. The platforms consist of three categories of stents: bare-metal stents (BMS), DESs, and bioresorbable stents. Every stent platform has distinct advantages, limits, and hazards, including the potential for stent fractures, which significantly affect the stent’s efficacy.6,7
BMSs are predominantly constructed from stainless steel, cobalt-chromium, or platinum-chromium alloys. The design consists of a basic iron framework lacking any further medicinal coating. The benefits of employing quick mechanical support to avert vascular collapse during angioplasty include decreased costs and an uncomplicated design.8 However, it has disadvantages, including the increased likelihood of restenosis, which is a re-narrowing of the artery caused by tissue regrowth referred to as intimal hyperplasia. Nonetheless, there exists a possibility of SF. SFs are less common with contemporary BMSs than with DESs; a 2022 meta-analysis of 36 studies (39,953 patients) reported pooled DES-related fracture rates of 5.5% per patient, 4.8% per lesion, and 4.9% per stent, with the incidence rising over the past 20 years,6 especially in long stents or in vessels that move substantially, such as the RCA. Cobalt-chromium BMSs exhibit greater flexibility and reduced susceptibility to fractures compared to earlier stainless steel BMSs. This can be attributed to the improved strength-to-weight ratio and thinner struts.9
DESs are typically comprised of cobalt-chromium, platinum-chromium, or newer alloys, such as titanium-nitride-oxide. Furthermore, a polymer is incorporated that elutes drugs (such as paclitaxel, sirolimus, everolimus, or zotarolimus) to impede tissue renewal and reduce restenosis. The advantage of drug elution in DESs is that it significantly diminishes the incidence of restenosis compared to BMSs by reducing smooth muscle cell proliferation. This enhances therapeutic outcomes, especially in high-risk patients with complicated lesions. However, delayed endothelialisation may result in late stent thrombosis, necessitating an extended course of dual antiplatelet therapy to reduce the risk of thrombus development.10
The incidence of SFs and their therapeutic implications have evolved with the advancement of newer-generation stents.
Initial Generation of the DES Sirolimus-Eluting Stents (SES)
The fracture rate of first-generation stents, such as the Cypher stent (Cordis Corporation, Hialeah, Florida, USA), was elevated, ranging between 2–9%11 due to their closed-cell design. This architecture rendered them less adaptable and more vulnerable to mechanical stress in regions of vascular flow.12 The incidence of stent fractures in Cypher stents showed a significant association with ISR and stent thrombosis, resulting in adverse clinical outcomes. A study indicated that all reported SFs were linked to SES and were associated with binary restenosis and target lesion revascularisation (TLR).13 Factors such as vessel tortuosity, overlapping stents, and placement in highly mobile channels, particularly the RCA, significantly elevate the likelihood of SFs.1
Paclitaxel-eluting stents, such as the Taxus stent (Boston Scientific), deliver paclitaxel to inhibit the restenosis of blood arteries. The Taxus stent, a first-generation DES, demonstrated a reduced but still notable incidence of SFs relative to the Cypher stent, with rates ranging between 0.5–1.5%.11 The Taxus stents exhibited enhanced pliability due to their open-cell architecture, leading to a reduced incidence of fractures, though not completely eradicating them.14
The Importance of Fractures in Clinical Practice
Fractures of first-generation DESs often led to ISR. SFs exacerbate this issue, especially in instances involving extensive lesions or arterial segments subjected to considerable stress. Stent thrombosis denotes the development of a thrombus within the stent, which can potentially lead to a life-threatening myocardial infarction. TLR frequently necessitates further procedures, such as balloon angioplasty or the placement of supplementary stents, as a result of fractures.15,16
Second-generation DESs, exemplified by the cobalt-chromium everolimus-eluting Xience platform, show markedly lower fracture rates than first-generation DESs. A large angiographic series reported SFs in roughly 2.9%17 of lesions, thanks to the thinner struts and more flexible design of these devices. Studies have shown that the occurrence of SFs in Xience stents is somewhat lower than in Cypher or Taxus stents.18,19 Fractures in everolimus-eluting stents (EES) might still lead to restenosis and require TLR; however, the latest stents demonstrate a reduced incidence of restenosis relative to their predecessors.20, 21
The fracture incidence of zotarolimus-eluting stents (ZES), including Medtronic’s Endeavour (1–2%) and Resolute (0.8–4.0%)22 models, demonstrates superior durability in intricate and convoluted blood vessels due to the open-cell structure and adaptability of the ZESs. Reports indicate fractures, particularly in extended or overlapping stents within the RCA.23,24 Similar to EESs, fractures in ZESs may lead to restenosis and sometimes necessitate revascularisation. A recorded case indicated that a ZES fracture led to ISR, which was successfully addressed with further angioplasty.25
Determinants that elevate the probability of SFs in second-generation DESs include: 1) geographical position, as the placement of stents in highly mobile arteries, such as the RCA, remains a considerable risk factor for fractures, similar to first-generation stents; 2) the length of the stent and overlap, as the utilisation of longer stents and overlapping configurations increases the mechanical stress on the stent, consequently augmenting the likelihood of fractures; and 3) complex lesions, particularly those involving angulated or tortuous arteries, have an elevated risk of SFs, irrespective of the stent generation.26-29
Finally, bioresorbable stents are constructed from bioresorbable materials such as poly-l-lactic acid or magnesium alloys. These provide temporary structural support and subsequently degrade, which potentially averts late-stage complications such as stent thrombosis. The stent fully dissolves after offering temporary support to the blood vessel, allowing the artery to regain its natural function. It may reduce the probability of long-term problems such as delayed stent thrombosis or ISR. Nonetheless, the disadvantages include heightened vulnerability to premature fractures owing to the inferior material properties compared to metallic stents.30-32
First-generation bioresorbable stents, exemplified by ABSORB (Abbott), had issues pertaining to strut thickness. This led to heightened chances of SFs and restenosis. The market removal of the ABSORB Bioresorbable Vascular Scaffold (BVS), Abbott, was necessitated by the emergence of these complications. There is a considerable probability of fractures arising in early bioresorbable stents, especially in the first-generation ABSORB stents, where a fracture rate of 1.3–8.4% was reported.33 Fractures predominantly manifested in regions of elevated stress or vascular mobility, which is attributable to the amalgamation of robust struts and inferior materials.34-36
Researchers are presently engaged in the development of advanced bioresorbable stents, including those made from magnesium. The objective of these stents is to reduce the probability of fractures. Nonetheless, they remain in the experimental phase and have not been extensively adopted in medical practice.37,38
The pathophysiological mechanisms that lead to SF are multifactorial. Fractures may be caused by mechanical stresses such as twisting, compressing, and stretching, which occur during cardiac cycles. Stents placed in arterial segments that are already under a lot of mechanical stress are particularly vulnerable. This is why platinum-chromium alloy stents, developed to improve radial strength and flexibility compared to earlier generations, potentially reduce the risk of SF.16
We can classify coronary SFs based on their degree of severity, anatomical site, and fracture pattern. Type I, which is the simplest form of SF, involves only one strut and may not have significant clinical consequences. In Type II, the fracture of multiple struts occurs without any separation between the stent segments. Type III involves the fracture of multiple struts with separation between stent segments, and can lead to more serious complications such as ISR or stent thrombosis. In Type IV, the stent has a transverse break, leading to complete division into two parts. This can be a serious complication with a significant clinical impact. Lastly, in Type V, the stent maintains its continuity but undergoes deformation, potentially altering the flow dynamics within the stent. These fractures have varying incidence and clinical impact, with more complex fractures (Types III and IV) associated with higher rates of adverse cardiac events.39
SF is the result of a complex interplay between biomechanical stresses and material fatigue. Repetitive mechanical stress, whether due to physiological vessel movement or external compression, can lead to metal fatigue and eventual fracture. The type of stent material and design also plays a critical role, with certain alloys and thinner strut designs being more susceptible to fracture. Hinged points in the artery, long stented segments, the RCA location, and metal overlap all represent contributing factors to SF.39-41
Coronary SFs have a range of clinical consequences, from asymptomatic cases detected incidentally on imaging to significant events such as ISR, thrombosis, and acute myocardial infarction. The presence of an SF increases the risk of adverse cardiac events, underscoring the importance of early detection and management.42,43
The diagnosis of coronary SF primarily relies on imaging modalities, including coronary angiography, IVUS, and optical coherence tomography (OCT). Each modality has its advantages and limitations regarding sensitivity and specificity for detecting SFs. Recent advancements in imaging technologies have improved the detection rates of these fractures.44-47
The management of coronary SF depends on the severity of the fracture, associated symptoms, and the presence of concomitant ISR or thrombosis. Options range from clinical observation for patients who are asymptomatic to percutaneous interventions or even coronary artery bypass grafting in cases with significant associated complications.7,48
Preventive strategies focus on minimising the risk factors for SF, including optimising stent selection based on vessel size and anatomy, avoiding excessive stent length and overlapping stents, and using imaging guidance to ensure optimal stent deployment. Advances in stent technology, including the development of stents with improved flexibility and fracture resistance, also contribute to reducing the incidence of SF.49,50
Ongoing research aims to further understand the mechanisms behind SFs, develop more resilient stent materials and designs, and improve diagnostic techniques for early detection. Although their role in preventing SFs is not fully elucidated, the advent of bioresorbable vascular scaffolds presents a potential avenue for reducing the long-term risks associated with permanent metallic stents.51,52
THERAPEUTIC INTERVENTION
The previously deployed stent at the mid-segment showed a complete SF. The SF occurred a few millimetres away from the overlapping segment, which was 2 mm in length itself. The minimum lumen area was measured at 7.02 mm². After changing the diagnostic catheter with a 6F Judkins Right (JR) 4.0 guiding catheter, the patient underwent IVUS-guided PCI to the RCA. Initially, an Onyx Frontier DES 3×18 mm was deployed, followed by a covered stent (PK Papyrus® [BIOTRONIK] 3.0×15 mm), deployed at 16 atm, achieving Thrombolysis in Myocardial Infarction (TIMI) flow Grade 3 end results (Figure 2). The authors started by deploying one DES to repair the disintegrated and fractured segment. Although the immediate result was still not satisfactory, given that contrast extravasation remained even after taking conservatory steps, the authors felt it would be safer to tackle this problem immediately. After deploying the covered stent, there was no more extravasation. Post-procedure minimal stent area was 9.72 mm² (Figure 3).
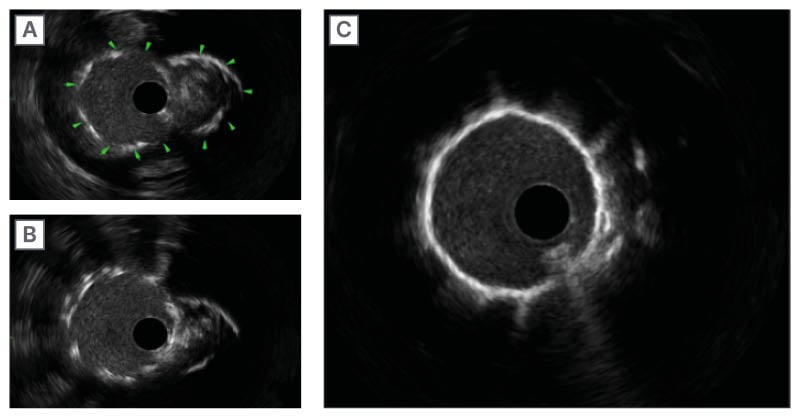
Figure 2: Intravascular ultrasound sequence demonstrating a double-lumen stent fracture and its resolution with drug-eluting stent-plus-covered-stent therapy.
A) Arrows show SF and dislocation resembling double lumen. B) SF site after deploying the DES. C) SF site showing single lumen after deploying the covered stent.
DES: drug-eluting stent; SF: stent fracture.
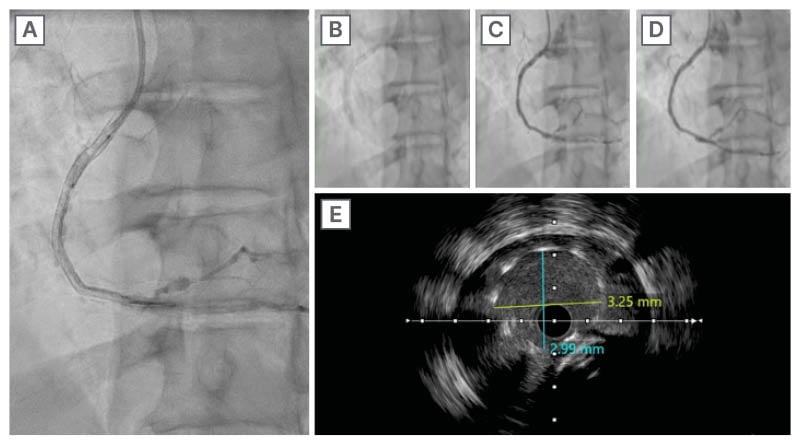
Figure 3: Serial right coronary artery angiograms.
A), B), C), and D) show serial RCA angiography after stent deployment with TIMI flow Grade 3. E) IVUS showing minimal stent area of 9.72 mm2.
IVUS: intravascular ultrasound; RCA: right coronary artery; TIMI: thrombolysis in myocardial infarction.
FOLLOW-UP AND OUTCOMES
The repeat PCI and deployment of an additional DES and a covered stent was followed by post-dilation with a Sapphire II NC™ balloon (OrbusNeich, Hong Kong, China; size 3.5×15 mm) up to 18 atm. The patient was also closely monitored during his hospital stay. Important follow-up diagnostics included an echocardiogram, which confirmed the absence of pericardial effusion, and IVUS imaging, which showed a satisfactory post-procedure minimal stent area of 9.72 mm², indicating the successful restoration of vessel patency.
The patient’s adherence to optimised medical therapy was assessed through regular consultations and medication reviews. He was compliant with the prescribed dual antiplatelet therapy (aspirin 100 mg daily and ticagrelor 90 mg twice daily), antihypertensive medication (perindopril 5 mg daily), lipid-lowering agents (atorvastatin 40 mg daily and evolocumab 140 mg every 2 weeks), and heart rate control medication (bisoprolol 2.5 mg daily). Tolerability was excellent, with the patient reporting no adverse effects from the medications during follow-up visits.
There were no adverse or unanticipated events during the follow-up period. The patient remained asymptomatic, with no episodes of chest pain or exertional dyspnoea. Routine follow-up angiography performed 2 months later demonstrated maintained vessel patency without evidence of restenosis or new SFs. The patient continued to abstain from smoking and adhered to lifestyle modifications aimed at improving his cardiovascular health.
DISCUSSION
Reports indicate that the incidence of SFs in EESs ranges from 0.5–2.9% of lesions and is a significant risk factor for major adverse cardiac events, primarily due to increased TLR and stent thrombosis.50 Factors such as stent length, vessel movement, and lesion calcification influence SF more frequently in certain anatomical locations, such as the RCA.45 In the authors’ case, the most likely causes of SF were the length of the stent and its location on the RCA’s hinge point.
The long-term results of clinical trials show that scaffold fracture after EES placement increases the risk of major adverse cardiac events and TLR, especially in the first year after implantation. However, the occasional peri-stent contrast staining after DES implantation does not appear to be associated with negative outcomes,52,53 a finding that was also observed in the authors’ case.
In terms of clinical management, SF is primarily detected through angiographic follow-up and fluoroscopy, especially with StentBoost,54, 55 IVUS, and OCT. These advanced imaging techniques provide detailed information about stent integrity, the level of fracture, and the potential impact on vessel patency. This information plays a crucial role in guiding subsequent management decisions.56 In the authors’ case, the angiographic appearance suggested the presence of SF, which was then confirmed by StentBoost and IVUS.
When coronary SFs are detected, the clinical context and the extent of the fracture determine the treatment options. Conservative management is possible for minor asymptomatic fractures, which may not require immediate intervention. However, more severe fractures necessitate intervention to address the underlying pathology and restore adequate blood flow.57,58 In the authors’ case, they observed the SF during routine follow-up, even though the patient was asymptomatic and had a complete transverse fracture with dislocation.
Among the possible treatments are stent repositioning, stent overlap, extra stent placement to improve stent deployment,56,57 or balloon angioplasty with drug-eluting balloons that deliver antiproliferative drugs without adding another layer of metal, which might lower the risk of breaking the stent again.59 In this case, the authors initially deployed a second DES that overlapped with the two parts of the fractured and dislocated segments. They then placed an additional covered stent at the site of the fractured stent due to concerns regarding the formation of an aneurysm or coronary perforation, especially since there was still extravasation staining at the site of SF.57 In complex cases where the SF is extensive or associated with additional complications, such as aneurysm formation, coronary artery bypass grafting may be considered. This is particularly relevant in cases where percutaneous approaches are not feasible or have failed.41
By carefully evaluating the lesion, selecting the appropriate stent, implementing optimal stent placement techniques, and potentially utilising imaging guidance such as IVUS to ensure proper stent fit and expansion, one can prevent SF. Moreover, patient-specific factors such as optimising medical therapy, especially antiplatelet therapy, are crucial for preventing thrombotic complications associated with SF.58
Continuous follow-up is crucial for patients with SF. This includes clinical evaluation; imaging studies, including coronary angiography to monitor the stent and coronary artery; and adherence to a strict regimen of antiplatelet therapy to prevent thrombotic events.59,60
While we focus on patients CTO due to their longer stent segments and higher stent complexity (which predisposes them to fractures), vigilant follow-up imaging can also benefit many high-risk patients who have undergone PCI.39 In general, a follow-up angiogram or non-invasive imaging at 3–6 months is advisable in complex CTO cases. For standard-risk patients, decisions can be individualised based on clinical presentation. At 6–12 months, the use of imaging such as IVUS, OCT, or advanced fluoroscopic techniques can detect silent fractures before they manifest clinically.39
The authors advise patients to make healthy lifestyle changes and manage risk factors for coronary artery disease, such as quitting smoking and managing blood pressure, cholesterol, and diabetes. This will improve their overall cardiovascular health and prevent future coronary events. In this case, the authors optimised the patient’s medications before discharge.60
CONCLUSION
SF is a rare but significant complication of PCI that can lead to adverse clinical outcomes such as ISR, stent thrombosis, and acute coronary syndromes. This case underscores the importance of early SF detection through routine follow-ups using advanced imaging modalities, such as intravascular ultrasound and OCT, which are critical for confirming stent integrity and guiding management decisions. In this instance, a Type IV fracture was identified during follow-up angiography in a patient who was asymptomatic. It was effectively managed by deploying a second DES and a covered stent to restore vessel patency and prevent complications such as restenosis and coronary perforation. The case highlights key risk factors for SF, including stent length, overlapping stents, and placement in highly mobile or calcified arterial segments. It reinforces the importance of personalised treatment planning, appropriate stent selection, and optimised medical therapy, including dual antiplatelet therapy, to minimise the risk of SF. While advancements in stent technology have reduced fracture incidence, continuous follow-up and lifestyle modifications, such as smoking cessation and better management of cardiovascular risk factors, remain crucial for improving patient outcomes.
PATIENT PERSPECTIVE
The patient was initially surprised to learn about the stent fracture during his routine follow-up, especially since he had been asymptomatic. However, he expressed relief that the issue was detected early before it could lead to more serious complications. He appreciated the clear explanations provided by his medical team about the nature of the complication and the steps needed to address it.
After the successful repeat PCI, the patient felt reassured by the prompt and effective treatment he received. He recognised the importance of adhering to his medication regimen and was motivated to make the recommended lifestyle changes, such as quitting smoking and managing his hypertension. The experience reinforced his commitment to regular medical follow-ups and proactive management of his cardiovascular health.