BACKGROUND
Infectious complications in those admitted to the ICU are a major contributor to mortality. Such nosocomial infections have been associated with the loss of commensal organisms and increased pathobionts in the gut. However, the relative contribution of different antibiotics to the disruption of intestinal microbiota in the ICU is poorly understood. The authors hypothesised that antibiotics with activity against butyrogenic bacteria are a principal contributor to altered microbiota composition.
METHODS
One hundred and ten mechanically ventilated ICU patients were recruited across two hospitals. Gut samples were collected longitudinally approximately every 48 hours, resulting in 397 samples, and 16S ribosomal RNA amplicon sequencing was performed. The impact of antibiotic use (grouped according to anaerobe coverage) was assessed in relation to microbiota alpha-diversity and the relative abundance of butyrogenic obligate anaerobes as well as pathobionts. Analysis was performed using generalised mixed-model regression, and adjusted for duration of stay, admission diagnosis, Sequential Organ Failure Assessment (SOFA) score, demographic characteristics, sample type, and medications.
RESULTS
ICU patients had a progressive depletion of butyrogenic obligate anaerobes and an enrichment of pathobionts, reflected in an associated decline in microbiota diversity. Cumulative exposure to antibiotics with anti-anaerobe activity (piperacillin/tazobactam, amoxicillin/clavulanic acid, metronidazole, and meropenem) was independently associated with a decline in butyrogenic taxa (4.01% relative abundance decrease with every additional 48 hours of antibiotics; 95% CI: 2.25%–5.84%; p<0.0001) and an increase in pathobiont prevalence (6.98% increase; 95% CI: 4.07%–9.89%; p<0.0001; Figure 1). The expansion of a single pathobiont to >30% of the microbiota was also positively associated with antibiotics with anti-anaerobe activity (odds ratio: 2.32; 95% CI: 1.35–4.01; p=0.0026). The most common pathobionts at >30% abundance included the genera Enterococcus, Streptococcus, and Escherichia-Shigella. In contrast, cumulative exposure to antibiotics with limited activity against anaerobes (intravenous vancomycin, ceftriaxone, cefepime, azithromycin, cefazolin) showed no significant relationship with abundance of either butyrate producers (estimate: -1.60%; 95% CI: -3.84%–0.55%; p=0.14) or pathobionts (estimate: 2.46%; 95% CI: -1.11–6.04; p=0.18; Figure 1).
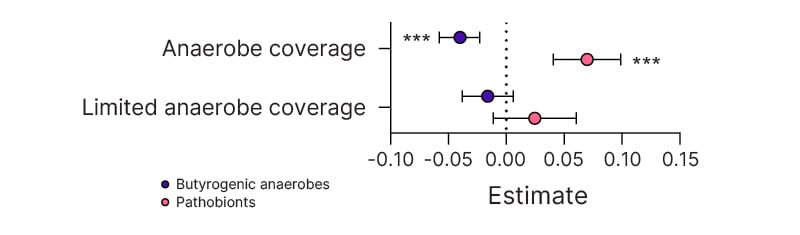
Figure 1: Effect of antibiotic exposure (either antibiotics that cover anaerobes or antibiotics that have limited coverage of anaerobes) on relative abundance of butyrate-producing anaerobes and pathobionts.
Estimate and 95% CI from generalised linear mixed models.
***p<0.0001.
CONCLUSION
Use of antibiotics with anti-anaerobe activity was associated with a loss of important commensal gut microbes and an increase in pathobionts. Alternative antibiotic options that preserve commensal anaerobes may reduce the risk of disseminated infections, such as sepsis, involving gut pathobionts.







