OBJECTIVES
This study evaluated the efficacy and safety of a dual therapy regimen comprising double-dose dolutegravir plus lamivudine in treating patients with HIV and tuberculosis.1
METHODS
This was a randomised, open label, parallel-controlled trial conducted at 16 centres in China (ChiCTR2300075493). It included adults with HIV who were antiretroviral therapy (ART)-naïve and had initiated rifampicin-based anti-tuberculosis therapy ≤2 weeks prior. Participants were randomised (1:1) to receive either dolutegravir (50 mg twice daily) plus lamivudine (two-drug regimen [2DR] arm), or efavirenz (600 mg daily) with tenofovir and lamivudine (three-drug regimen [3DR] arm), beginning at 2 weeks after anti-tuberculosis treatment. The primary outcome and secondary outcome were virological suppression (HIV-1 RNA <50 copies/mL) at Week 24 in the modified intention-to-treat (mITT) population (defined as receiving at least one dose of ART drugs according to the protocol) and in the per-protocol-set (PPS; defined as strictly adhering to the protocol without major deviations).
RESULTS
Between July 2023–March 2025, 88 participants belonging to the mITT exposed population were randomly allocated to and received at least once either the 2DR (N=48) or 3DR (N=40).
Forty-nine participants (29 in the 2DR arm and 20 in the 3DR arm) finished Week 24 visits, of which 38 participants belong to the PPS (25 in the 2DR arm, and 13 in the 3DR arm). In the mITT set completing Week 24 visits, for the 2DR group 41.4% (12/29) had baseline HIV RNA ≥500,000 copies/mL. Mean baseline HIV-1 RNA: 5.5±0.64 log10 copies/mL. Median CD4+ count: 46 cells/μL (interquartile range: 29.3–130). For the 3DR group, 20% (4/20) had HIV RNA ≥500,000 copies/mL. Mean baseline HIV RNA: 5.2±0.76 log10 copies/mL. Median CD4+ count: 59 cells/μL (interquartile range: 13.8–84.2). At Week 24, virological suppression rates were 62.1% (18/29; 95% CI: 43.3–80.9) versus 70% (14/20; 95% CI: 48–92) in the 2DR and 3DR group for the mITT population, and 72% (17/25; 95% CI: 53.1–90) versus 100% (13/13; 95% CI: 100–100) in the 2DR and 3DR group for PPS analysis (Figure 1), respectively. There was no statistically significant difference in viral suppression (p=0.566 for mITT and p=0.072 for PPS). For those with baseline viral load ≥500,000 copies/mL, the suppression rates of Week 24 for the mITT set were 50% (6/12) (95% CI: 16.8–83.2) versus 75% (3/4) (95% CI: 0–100) in the 2DR and 3DR group (p=0.691); the suppression rates of Week 24 for the PPS set were 60% (6/10; 95% CI: 23.1–96.9) versus 100% (3/3; 95% CI: 100–100) in the 2DR and 3DR group (p=0.497). Twelve immune reconstitution inflammatory syndrome events were reported: nine in the 2DR group and three in the 3DR group, with no discontinuations due to the syndrome. There were two severe adverse events, but neither were directly related with ART drugs.
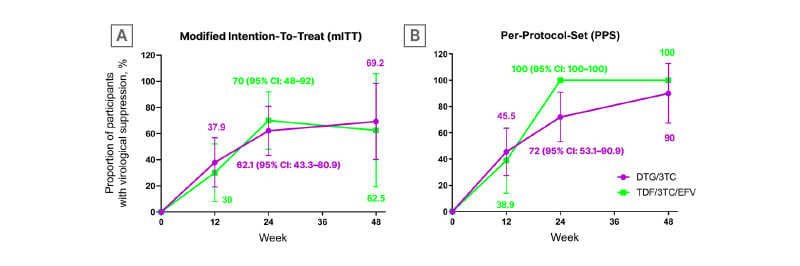
Figure 1: Proportion of participants with HIV-1 RNA <50 copies/mL over time in modified intention-to-treat (A) and per-protocol set (B) analysis.
This analysis presents the preliminary results of the study, with only a small number of patients having completed the 48-week follow-up; this study is still ongoing with continued enrollment and follow-up of patients.
DTG/3TC: dolutegravir/lamivudine; TDF/3TC/EFV: tenofovir/lamivudine/efavirenz.
CONCLUSION
Double-dose dolutegravir with lamivudine was equally effective for adults with HIV who were on rifampicin-based anti-tuberculosis treatments compared to a three-drug regimen of efavirenz, tenofovir, and lamivudine. This dual therapy may provide a new option for patients with HIV and tuberculosis.







