BACKGROUND
Over 10% of antibiotic prescriptions are for skin infections.1,2 New therapeutic approaches are required, and new modalities may be favourable so that they are not undermined by currently circulating resistance mechanisms. Epidermicin NI01 is a first-in-class bacteriocin with potent activity and a novel mode of action against Staphylococci (including methicillin-resistant Staphylococcus aureus) and Streptococci,3 the leading causes of skin infections.4
METHODS
Male CD1 mice (n=6 per group) were immunosuppressed (cyclophosphamide; 150 mg/kg and 100 mg/kg on Days -4 and -1, respectively). Dorsal skin (1×2 cm) was shaved and hair was removed, before three rounds of skin tape-stripping and topical application (2×106 CFU/mouse) of S. aureus strain USA300, a well-recognised cause of community-acquired skin infections.5 Topical administration every 24 hours (q24h) of 50 μL of vehicle (0.5% hydroxypropyl methylcellulose [HPMC]) or NI01 (four dosing regimens in 0.5% HPMC), or 50 mg of mupirocin 2% or fusidic acid 2%, which are standard for the treatment of impetigo (according to the National Institute for Health and Care Excellence [NICE] guidelines),6 was initiated 24 hours post-infection. Weight monitoring, general health assessment, and skin clinical scoring were conducted daily until the endpoint at 96 hours, when quantitative skin burden (CFU/g) analysis was carried out.
Ex vivo human skin was recovered during ‘tummy tuck’ surgery, and a bolus of NI01 or control compound Substance P, which is known to stimulate histamine release from mast cells,7 was introduced subdermally. An anti-Immunoglobulin E antibody was used, as this leads to an antibody-meditated histamine release. Histamine levels were measured in a PIPES buffer that was perfused through the skin using semi-permeable tubing (Figure 1A).
All histamine concentration data was compared to a PIPES buffer control using a two-way analysis of variance with Dunnett’s comparison test.
RESULTS
There was no marked weight loss, and there were no general signs of illness or adverse effects due to infection or treatment in any group. No efficacy was seen in the 3% NI01 mono-dose group or in groups treated q24 with 0.5% or 1% NI01. Some treatments resulted in significant log10 reductions in CFU/g tissue: 2.82 for fusidic acid q24; 2.48 for 3% NI01 q24; and 2.47 for mupirocin q24. The minimum inhibitory concentration of NI01 for S. aureus strain USA300 was 4 μg/mL before the study and ex vivo, indicating no change in susceptibility as a result of exposure to epidermicin NI01 during the study.
Histamine levels, which indicate allergic impacts during topical application, were low in all NI01 groups, at concentrations up to 80 μg/ml, approximately 20 times the minimum inhibitory concentration ([hlFigure 1B[/hl]). This indicates the unusual activity of NI01, which does not have the liabilities seen with compounds like Substance P.
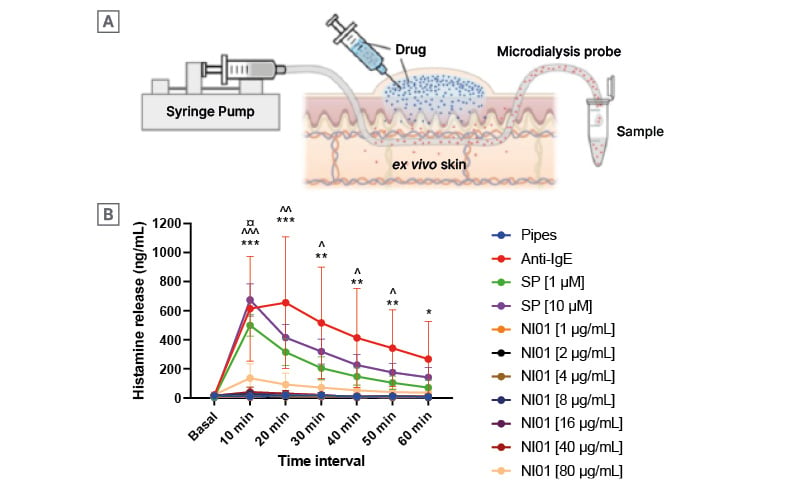
Figure 1: Measurement of histamine release in ex vivo human skin exposed to epidermicin NI01 and control compounds Substance P and anti-IgE antibody.
Measurement of histamine release in ex vivo human skin exposed to epidermicin NI01 and control compounds Substance P and anti-IgE antibody. Test material was introduced as a sub-cutaneous bolus in the skin (A) and buffer perfused through semi-permeable tubing and collected for analysis. Histamine levels were measured in perfusate over a 60-minute period (B). All data points for NI01 were compared to Pipes using a two-way ANOVA with Dunnett’s comparisons test and the statistical difference is indicated using the key below.
€: different to anti-IgE; ^: different to SP [1 μM]; *: different to SP [10 μM]; *, **, and *** indicate a level of probability of 0.05, 0.01, and 0.001, respectively. SP: Substance P; NI01: epidermicin NI01.
CONCLUSION
These data warrant further development of NI01 for use in the topical therapy of infections caused by the WHO priority pathogens, and validate expansion of our AI-driven discovery pipeline for drugs in the epidermicin class to treat drug-resistant infections.