Abstract
Background: Advanced healthcare facilities have improved the survival of preterm babies and critically ill or immuno-compromised children. However, they have simultaneously increased the incidence of opportunistic infections like candidiasis.
Materials and methods: This is a 3-year retrospective descriptive study from the paediatric intensive care unit (PICU) and neonatal intensive care unit (NICU) of a tertiary care hospital in South India. The authors retrieved data from culture-proven candidiasis in blood, urine, and other fluids from January 2019–December 2021 to identify causative species, antibiotic sensitivity patterns, associated risk factors, and patient outcomes.
Results: Out of 1,843 admissions, 276 patients had culture-proven infections; among them, 22 (1.12%) were Candida. The incidence of Candida infection was 0.7% and 1.4% in the NICU and PICU, respectively. Out of 22 candidiasis cases, 77.2% were from the PICU and 22.7% from the NICU. Candida albicans and Candida tropicalis were the most common isolates in the NICU and PICU, respectively, and they were sensitive to fluconazole, other azoles, and echinocandins. Predisposing risk factors included the presence of invasive lines (77.3%), prior antibiotic exposure (95%), and surgical intervention (10%). A total of 54.5% of cases had bacterial co-infection during management. Overall mortality was 22.7%, with 20% and 23.5% cases from the NICU and PICU, respectively. All of these patients had multiple comorbidities, and some had serious bacterial co-infections.
Conclusion: C. albicans and C. tropicalis are the commonest species responsible for invasive Candida infections, which are highly sensitive to fluconazole. These infections are almost always associated with risk factors, which is why high suspicion and early management are key to achieving a better outcome in such cases.
Key Points
1. Candidaemia causes significant morbidity and mortality in paediatric and neonatal ICUs. Prompt diagnosis, treatment, and prevention are critical, especially with rising antifungal resistance and high-risk factors like invasive lines and antibiotics.2. In this retrospective ICU study, the authors found a candidaemia incidence of 1.12%, mostly involving Candida tropicalis and Candida albicans, with 22.7% mortality. Major risk factors were intravenous antibiotics and invasive lines.
3. Early antifungal treatment, as well as limiting invasive devices and inappropriate antibiotic use, are critical to reduce Candida-related ICU mortality. Fluconazole remains first-line, but resistance requires species identification and antifungal stewardship.
INTRODUCTION
Invasive Candida infections represent a significant healthcare concern, particularly in the vulnerable paediatric population. Candida species, a group of fungi normally present in the human microbiota, can cause invasive infections when they breach the body’s natural defence mechanisms.1,2 In recent years, the incidence of invasive Candida infections in children has been on the rise, leading to increased morbidity and mortality rates. Candida is the most common invasive fungal infection. The incidence of invasive Candida infection is higher in children and particularly newborns than in adults.3,4 It is one of the commonest organisms causing bloodstream infections in neonatal and paediatric patients and is associated with a very high fatality rate, with an approximate mortality of 30–50%.5–14 The incidence of candidaemia is 4–15 times higher in developing countries compared to developed ones.15 The average incidence of Candida infection in developed and developing countries varies from 0.03–1.86 per 1000 admissions and 0.26–4.2 per 1000 admissions, respectively.15 In India, the incidence of hospital-acquired candidaemia in ICU patients is 6.51 cases per 1,000 admissions.16,17
Invasive Candida infections encompass a spectrum of diseases, ranging from bloodstream infections (candidaemia) to deep-seated organ involvement. These infections are associated with a wide range of clinical presentations, making early detection and prompt intervention critical for optimal patient outcomes. The most commonly implicated Candida species in paediatric cases include Candida albicans, Candida parapsilosis, Candida tropicalis, Candida glabrata, and Candida krusei.18,19 Because of advanced healthcare, there is an improvement in the survival of critically ill and immune-compromised patients. However, this is associated with increased opportunistic infections like candidiasis. Candidaemia is also associated with other risk factors such as invasive lines, prolonged antibiotic courses, children on total parenteral nutrition, and H2 blockers, as well as recent major surgery, particularly abdominal, necrotising pancreatitis, or peritoneal dialysis. Low birth weight children and necrotising enterocolitis (NEC) are also risk factors for preterm newborn babies.20–22
Treating invasive Candida infections in the paediatric age group poses unique challenges. Antifungal therapy with agents such as fluconazole, amphotericin B, or echinocandins is typically employed, taking into consideration factors such as the severity of the infection, patient age, immune status, and local patterns of antifungal resistance. Fluconazole and amphotericin B are usually effective against this organism, but with the emergence of multidrug-resistant Candida species, the availability of sensitivity reports and knowledge of local antibiograms are crucial for early intervention.11,13,23–27
For better management of patients, preventing drug toxicity, and antibiotic stewardship, knowledge of regional organisms causing infections and their sensitivity patterns is not only helpful for local practitioners but also important for policymakers in creating guidelines. This knowledge is essential for shaping policies that aim to improve patient outcomes. Additionally, management often involves a multidisciplinary approach, including close collaboration between infectious disease specialists, intensivists, surgeons, and other healthcare providers.
This study was conducted to identify the Candida species responsible for infections, assess their sensitivity to antifungals, investigate associated risk factors, and analyse outcomes in the neonatal intensive care unit (NICU) and paediatric intensive care unit (PICU) within the hospital setup. While numerous studies have explored this area, this research uniquely contributes data from South India, where limited published information is available. Moreover, the study not only encompasses Candida species and sensitivity but also explores risk factors and outcomes.
OBJECTIVE
The objective of this study is to investigate several key aspects related to invasive Candida infection in the paediatric population. The specific objectives are as follows:
Prevalence of species-specific invasive Candida infection: the primary aim is to determine the prevalence of different Candida species causing invasive infections in paediatric patients.
Sensitivity pattern of Candida infection: the study intends to analyse the sensitivity pattern of the identified Candida species to antifungal drugs. This will help guide appropriate treatment choices and determine the effectiveness of different antifungal agents.
Risk factors associated with invasive Candida infection: the study seeks to identify and evaluate risk factors associated with the development of invasive Candida infection in paediatric patients. Understanding these risk factors will aid in preventive strategies and targeted interventions.
Outcome of invasive Candida infection: the study aims to assess the outcomes of paediatric patients with invasive Candida infection.
METHODOLOGY
Materials and Methods:
This study utilised a retrospective descriptive design, involving the collection and analysis of data across 3 years, from January 2019–December 2021. It was a single-centre study conducted at a PICU and NICU of a multispecialty hospital located in Bengaluru, South India, after appropriate consent and ethical approval. A no-objection certificate (NOC) was issued by the institute, confirming that the publication of data complies with institutional policies and ethical standards. The study strictly adhered to ethical principles, including the anonymisation of patient data and the protection of confidentiality.
Data Collection:
A convenience sampling approach was used, which included all patients admitted to the PICUs and NICUs of the institute during the specified study period. Patients without any infection or those with infections unrelated to Candida were excluded from the study to ensure the accuracy and relevance of the data.
Methodology:
All available clinical data from paediatric and neonatal patients who were admitted to the ICUs during the designated study period were retrospectively collected and analysed. Specifically, patients suspected of septicaemia, presenting with clinical indications, had samples collected from various sources, including blood, urine, pus, or others. These samples were then cultured in the in-house microbiology laboratory.
The identification of microorganisms was performed using the BacT/ALERT 3D automated system (bioMérieux, France). The system aids in the detection and identification of microbial growth, enabling the identification of the Candida species responsible for the infections. Further species identification of the Candida isolates was performed using the Vitek 2 YST identification card (bioMérieux, France), which provides accurate identification based on specific characteristics of the isolates. Standard operating procedures, as described by the manufacturer, were followed.28– 30
Data collection focused specifically on patients with culture-positive Candida infections, allowing for comprehensive analysis. Detailed information regarding the species of Candida involved and their sensitivity to antifungal drugs was collected and studied. Additionally, relevant clinical parameters of the paediatric patients were documented. Risk factors associated with Candida infection, such as prolonged antibiotic use, presence of invasive lines, and underlying comorbidities, were assessed and compared across patients. Finally, patient outcomes, including mortality rates and other significant clinical parameters, were evaluated and compared to determine the impact of invasive Candida infection on the paediatric population.
RESULTS
Patients details and risk factors
Out of a total of 1,843 ICU admissions, 276 had culture-proven infections, of which 22 (1.12%) were positive for Candida. In the NICU, a total of 693 admissions were recorded, with 5 out of 75 culture-proven infections growing Candida. In the PICU, there were 1,150 admissions, with 17 out of 201 culture-proven infections growing Candida (Flowchart 1). The incidence rate of Candida infection was 0.7% in the NICU and 1.4% in the PICU, respectively. In contrast, 6.6% in the NICU and 8.4% in the PICU of culture-proven infections were due to invasive candidaemia. Female children were more affected than male children, with a male-to-female ratio of 1:4 in the NICU and 8:11 in the PICU. Of the 22 samples, 40% were isolated from blood, 40% from urine, and 20% from other fluids.
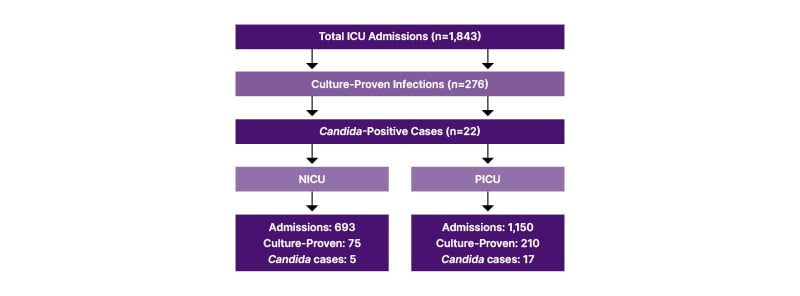
Flowchart 1: Patient selection and Candida infection identification.
NICU: neonatal ICU; PICU: paediatric ICU.
As mentioned in Table 1, 77.3% of cases had one or more invasive lines in situ before collecting culture samples. Except for one case, all other patients were either on intravenous (IV) antibiotics or had received IV antibiotics within 90 days prior to admission. Surgical intervention was required in 10% of cases. Among the 54.5% of cases with culture-proven bacterial co-infections during management, the most common was Klebsiella pneumoniae (seven cases), followed by Enterococcus (three cases), Escherichia coli (one case), and coagulase-negative Staphylococcus epidermidis (one case). In the NICU, 60% of infected newborns were preterm. Three out of five newborns had late-onset Candida infection (after 48 hours of life), while the remaining two had early-onset Candida sepsis.
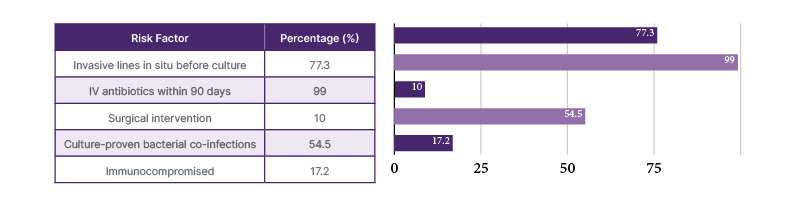
Table 1: Risk factors for invasive fungal infections among patients in the neonatal intensive care unit and the paediatric intensive care unit
IV: intravenous.
Microbiological findings
In the PICU, the most common species responsible for candidaemia was C. tropicalis (n=7, 41%), followed by C. albicans (n=4, 23%), C. famata (n=2, 12%), and C. parapsilosis (n=2, 12%). One sample grew Candida auris (6%), and in one sample, the species could not be identified. In the PICU, 15 out of 17 cases were sensitive to fluconazole. Fluconazole-resistant organisms included C. parapsilosis, which was managed with voriconazole, and C. auris, which was pan-drug resistant (Table 2).
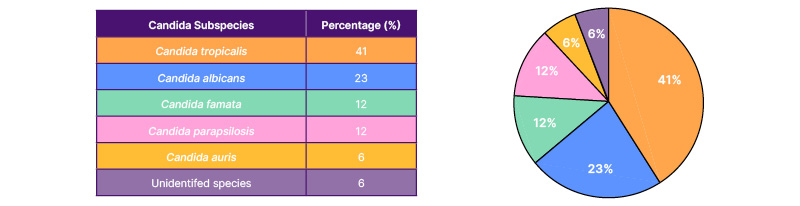
Table 2: Distribution of Candida subspecies causing invasive infections in the paediatric intensive care unit.
In the NICU, C. albicans was the most common species (n=2, 40%), followed by C. pelliculosa, C. glabrata, and non-albicans Candida species, which grew once out of five samples. Two out of five culture-positive Candida infections were resistant to fluconazole (one C. glabrata and one C. pelliculosa) (Table 3). All three fluconazole-sensitive cases were successfully treated with fluconazole for 14 days in the NICU. The fluconazole-resistant cases were managed with conventional amphotericin B. Unfortunately, a newborn infected with C. glabrata succumbed despite appropriate antifungal therapy (amphotericin B) due to meconium aspiration syndrome, sepsis, and acute kidney injury.
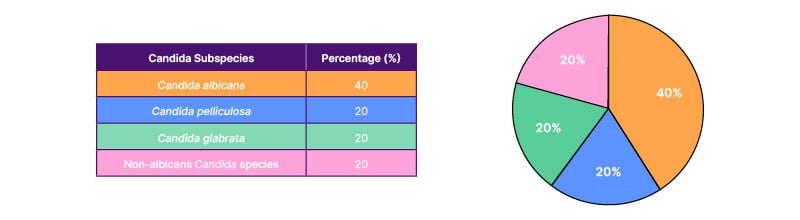
Table 3: Distribution of Candida subspecies causing invasive infections in the neonatal intensive care unit.
Outcomes
Overall mortality was 22.7% (5 out of 22) in culture-proven Candida infections. There was no statistically significant difference in risk factors when comparing patient outcomes (p>0.05), as shown in Table 4. Mortality was 20% (1 out of 5) in NICU cases and 23.5% (4 out of 17) in PICU cases. In blood culture-positive cases, mortality was 40% (4 out of 10). All four PICU cases had multiple comorbidities, such as inborn errors of metabolism (IEM), syndromic conditions, and chronic organ dysfunction. Three of the four had serious bacterial co-infections. One NICU case had severe sepsis along with multiple organ dysfunction syndrome (MODS). One NICU case was discharged on request while still being managed.
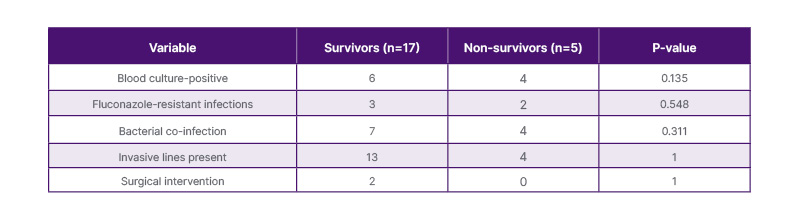
Table 4: Comparison between survivors and non-survivors in culture-proven Candida infections.
DISCUSSION
In this study, the incidence rate of culture-proven Candida infections was 0.01 per 1000 admissions, which is lower than the data from developing countries (0.26–4.2 per 1000 admissions).15 In culture-proven infections, candidiasis was the responsible organism in around 8% of cases (22 out of 276), which is double the rate found in the NeoOBS study on invasive Candida infections in low- and middle-income countries.15,31
In this study, females were more affected than males in both PICUs and NICUs, as seen in the NeoOBS study in neonatal ICUs, while a fungal infection study in the PICU in South India showed male dominance.31,32. In this NICU, 40% of cases were caused by C. albicans and 60% were due to non-albicans species. In the PICU, C. tropicalis was the most common species (41%), followed by C. albicans (23%). The predominance of C. albicans in NICU cases can be attributed to the underdeveloped immune systems of newborns, especially preterm infants, and its role as a common commensal. In contrast, PICU patients, being older, with complex comorbidities and greater exposure to healthcare interventions, are more prone to infections by non-albicans species like C. tropicalis, which are associated with healthcare environments. Regarding susceptibility, both C. albicans and C. tropicalis were susceptible to fluconazole. The overall susceptibility of Candida to fluconazole was 81.9%. All Candida species were sensitive to amphotericin B and voriconazole (95.5%), except C. auris, which was pan-resistant to all antifungals.
According to the recent NeoOBS study on neonatal invasive Candida infections, C. albicans emerged as the predominant cause of invasive fungal infections, accounting for 35% of cases, followed by C. parapsilosis at 30% and C. auris at 14%, particularly in low- and middle-income countries, including India, aligning with the authors’ own research findings.31 The study revealed that C. albicans isolates exhibited a sensitivity of 91% to fluconazole and 100% to amphotericin B. In contrast, notable resistance to fluconazole was observed in the majority of C. parapsilosis and C. auris, with 85% of C. auris isolates also showing resistance to amphotericin B and 31% to voriconazole. The overall sensitivity to fluconazole was 60%, and 82% to amphotericin B.31,33,34 The disparity in sensitivity observed in the authors’ study may be attributed to varying antibiotic usage policies and the avoidance of prophylactic antifungal drug use in their intensive care setup.
A 2016 research article from Kolkata, West India, examined 70 Candida-positive culture reports and reported findings similar to the authors’, with nearly 50% attributed to Candida albicans.35 Additionally, an Iranian study mirrored these results, indicating that 47.7% of invasive fungal isolates were C. albicans, followed by C. glabrata and C. parapsilosis. Notably, almost 100% sensitivity to fluconazole was observed in C. albicans, in C. parapsilosis, and nearly 90% in C. glabrata.36 Studies conducted in haematology units and PICUs on invasive fungal infections also affirmed that C. albicans is the most prevalent Candida infection in children.37–39 The authors’ study recorded a mortality rate of nearly 23% in paediatric patients with invasive fungal infections, consistent with other research findings.31,36 Patients with underlying comorbidities exhibited a higher mortality rate.37
In this study, the major risk factor was previous IV antibiotics, which were received by all patients except one. Among them, nearly 60% had culture-proven bacterial infections. Another risk factor was the presence of invasive lines, found in over 75% of patients, correlating with research by Rajeshwari et al.32 A study published by Hlophe et al.38 on 36 culture-proven paediatric invasive fungal infections from low- and middle-income countries showed that 100% of patients were on IV antibiotics and two-thirds had invasive lines, and nearly 44% of cases had a history of previous surgical intervention.38 Research from North India by Lamba et al.40 found 60% late-onset Candida septicaemia and 40% early-onset, with around 60% of newborns delivered preterm.
Earlier research by Kumar et al.41 examined Candida infections in children with onco-haematological malignancies in South India. The study found that C. albicans already exhibited notable resistance to fluconazole, and C. tropicalis was frequently identified as a causative agent of infections. In contrast to the authors’ findings, Kumar et al. found that 17% of cases were resistant to fluconazole.41
CONCLUSION
Candida infections are frequently encountered in ICUs, including NICUs and PICUs. Among the various Candida species, C. albicans and C. tropicalis are commonly isolated in cases of invasive candidaemia. Fortunately, the majority of invasive Candida infections are susceptible to treatment with fluconazole, making it the recommended empirical drug of choice when an invasive fungal infection is suspected, pending culture reports.
It is important to note that Candida infections are almost always associated with specific risk factors. Prolonged use of antibiotics and the presence of invasive lines, such as central venous catheters, are the most common risk factors for developing these infections. To minimise the risk, healthcare providers should exercise caution when considering the insertion of invasive lines, ensuring they are only used when necessary. Additionally, early removal of these lines when they are no longer essential is advisable. In preventing Candida infections, strict adherence to aseptic precautions is crucial to avoid hospital-acquired infections. This includes practicing proper hand hygiene, using appropriate barriers, and following sterile techniques during procedures. Maintaining a robust infection control program within the ICU setting is paramount to reducing the incidence of Candida infections.
Implementing regular antibiotic stewardship practices is also key to preventing unnecessary use of antibiotics within the ICU. This practice aims to optimise antibiotic use by ensuring that patients receive the right medication, at the right dose, and for the appropriate duration. By reducing the indiscriminate use of antibiotics, the risk of developing invasive fungal infections can be significantly decreased.
Early suspicion of fungal infection is also important, particularly when high-risk factors are present. Timely recognition and prompt initiation of antifungal therapy, such as fluconazole, can significantly improve survival rates and patient outcomes. Therefore, healthcare providers should maintain a high index of suspicion for fungal infections in patients with known risk factors, such as prolonged antibiotic use or the presence of invasive lines.
LIMITATIONS
This study has several limitations. It is a single-centre retrospective study, which limits the generalisability of the findings. To validate these results and obtain a more comprehensive understanding, multicentre studies with larger sample sizes are required. Additionally, the lack of a standardised clinical scoring system for the diagnosis of invasive Candida infection remains a challenge. Future research should focus on developing a valid scoring system for the early diagnosis and treatment of invasive Candida infection.







