Abstract
Introduction: HIV monitoring in resource-limited settings is often hindered by diagnostic barriers that compromise patient outcomes. Although viral load testing is the gold standard, cluster of differentiation (CD)4 count monitoring remains common due to cost and accessibility limitations.
Case Presentation: The author presents the case of a 38-year-old male in Ecuador who is
HIV-positive and undergoing long-term antiretroviral therapy. Despite sustained high adherence, the patient exhibited marked CD4 count variability. A sharp decline 23 months after initial diagnosis (Month 23), followed by a recovery 2 months later (Month 25), raised concerns over laboratory discrepancies and transient clinical conditions.
Discussion: The case highlights the multifactorial nature of CD4 variability, encompassing laboratory quality control, medication supply chains, and biological stressors. A review of
the literature supports the role of systemic challenges in such variability, especially in
low-resource settings.
Conclusion: Reliable immunological monitoring requires stringent diagnostic protocols, robust healthcare integration, and attention to clinical context, even in patients with stable antiretroviral therapy adherence.
Key Points
1. Laboratory quality control, regular calibration, and oversight of equipment are crucial to ensure reliable CD4 and viral load testing and prevent errors affecting patient outcomes.2. A unified global public health network is required to standardize diagnostics, reduce disparities, and foster collaboration between developed and developing countries.
3. Expanding insurance coverage for routine and external diagnostics, integrating specialized testing into social security coverage, and prioritizing comprehensive management of common illnesses in immunocompromised patients can improve holistic care and address systemic inefficiencies.
INTRODUCTION
The global management of HIV continues to face significant challenges, particularly in low- and middle-income countries, where diagnostic capacity and health system infrastructure often limit the effectiveness of treatment monitoring strategies.1-4 Although viral load (VL) testing is recognized as the gold standard for evaluating antiretroviral therapy (ART) efficacy and detecting treatment failure, its routine use is frequently hindered by cost, technological constraints, and limited accessibility in resource-limited settings.5-8 In such contexts, cluster of differentiation (CD)4 count monitoring remains widely utilized despite its lower specificity, providing a more accessible, albeit indirect, measure of immune system status and disease progression.9-12
However, CD4-based monitoring presents its own limitations. Variability in CD4 values can result from pre-analytical and analytical inconsistencies, biological fluctuations, or comorbid conditions, complicatingclinical interpretation.9,13,14
This case report presents the clinical course of a 38-year-old male in Ecuador who is HIV-positive and undergoing long-term ART. Despite high adherence and virological suppression, the patient experienced a marked, transient decline in CD4 count followed by spontaneous recovery. The episode raised concerns regarding laboratory accuracy, the impact of minor clinical events, and potential implications of medication formulation changes.3
By examining this case in the broader context of HIV care in resource-limited settings, the author aims to illustrate the multifactorial nature of CD4 variability and highlight the need for integrated diagnostic strategies, rigorous laboratory oversight, and coordinated public health systems. This report also contributes to the literature by discussing how individual patient outcomes intersect with systemic challenges in monitoring HIV, particularly in settings where VL testing remains inaccessible or delayed.
CASE PRESENTATION
Patient Information
A 38-year-old male healthcare professional from Ecuador was diagnosed with HIV after presenting for routine testing. At diagnosis, his CD4 count was 167 cells/μL and VL measured 105,156 copies/mL. He reported no prior opportunistic infections and had no history of intravenous drug use or high-risk sexual behavior beyond unprotected intercourse with multiple partners.
Medical History and Treatment Initiation
Shortly after diagnosis, the patient was initiated on a first-line ART regimen consisting of tenofovir disoproxil fumarate, lamivudine, and dolutegravir. The combination was initially dispensed by the Instituto Ecuatoriano de Seguridad Social (IESS) through a generic formulation manufactured by Mylan (acquired by Viatris, Canonsburg, Pennsylvania, USA). In mid-2024, a change in procurement policy led to a non-clinically justified switch to a formulation by Hetero (Hyderabad, India), also supplied via the Global Fund (Geneva, Switzerland). No adverse effects or clinical deterioration were noted following the change.
The patient reported excellent adherence throughout the treatment period, missing no more than one monthly dose. He also reported concurrent antidepressant therapy (sertraline), as well as a medical history of allergic rhinitis, irritable bowel syndrome, and central abdominal obesity.
Clinical Course and Laboratory Findings
During routine follow-up, the patient’s immunological and virological parameters were periodically assessed. Table 1 summarizes the progression of CD4 counts and VL measurements from initial diagnosis to Month 25. After an expected increase in CD4 count following ART initiation, the patient experienced a marked drop in Month 23 (406 cells/μL), despite maintaining full clinical stability and virological suppression. Two months later, in Month 25, his CD4 count rebounded significantly (759 cells/μL), although VL showed a marginally detectable level (<40 copies/mL).
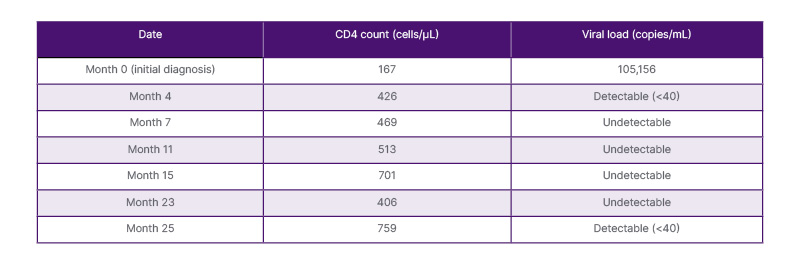
Table 1: Cluster of differentiation 4 count and viral load results over the course of treatment.
CD: cluster of differentiation.
This sequence raised concerns about the reliability of laboratory results. Notably, the sample from Month 23 was processed in a tertiary-level public hospital, while the sample from Month 25 was analyzed in a private laboratory. The divergence suggested possible analytical variation, technical inconsistencies, or the influence of intercurrent clinical conditions.
The patient denied any major symptoms prior to the Month 23 measurement. However, early in Month 25, he reported a brief episode of low-grade fever and a mild contusion to the lower limb, both of which resolved spontaneously. No ART interruptions or other clinical events were documented during the observed period.
Patient Perspective
The patient expressed concern regarding the inconsistency in laboratory results and the lack of immediate clinical explanation. He emphasized the need for greater transparency in laboratory practices and for patient-centered communication, especially when unexpected changes in key health indicators arise.
DISCUSSION
This case illustrates the diagnostic and interpretive complexities involved in HIV monitoring within resource-limited settings, even in the context of excellent ART adherence and apparent clinical stability. The observed CD4 count fluctuation, particularly the marked decline in Month 23 followed by rapid recoveryin Month 25 (Figure 1), raises critical considerations regarding laboratory reliability, biological variability, and systemic health system challenges.5
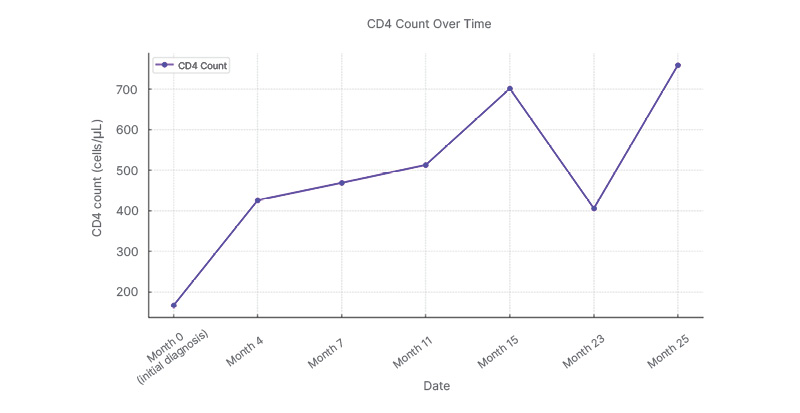
Figure 1: The patient’s cluster of differentiation 4 trajectory across the treatment timeline.
A general upward trend is highlighted, interrupted by the notable decline in Month 23 and subsequent recovery in Month 25. This visual representation emphasizes the transient nature of the immunological fluctuation.
CD: cluster of differentiation.
Technical and Laboratory Considerations
One of the most salient issues in this case relates to laboratory inconsistencies. As highlighted in previous studies, variability in CD4 count results across laboratories is a well-documented phenomenon, particularly in settings where quality control standards are unevenly implemented.9,13,14 The use of different testing centers (one public and one private) may have introduced pre-analytical or analytical discrepancies, such as differences in sample handling, instrument calibration, or procedural protocols. These concerns are supported by literature emphasizing the central role of rigorous quality assurance in both CD4 and VL monitoring, particularly in decentralized health systems.1,5,6,13
Biological and Clinical Influences
Beyond technical factors, transient clinical conditions can exert a measurable impact on immune markers. Although the patient was asymptomatic at the time of the test in Month 23, he reported a minor febrile illness and physical trauma early in Month 25. These events, albeit clinically insignificant, could have contributed to immune activation and short-term changes in CD4 distribution or turnover.15,16 The influence of low-grade infections and physical or psychological stress on CD4 counts has been previously documented, and must be considered in interpreting unexpected laboratory results.16,17
The patient’s underlying conditions, including abdominal obesity and the use of antidepressants, may also exert a subtle immunomodulatory effect over time. Weight gain associated with ART initiation has been linked to alterations in immune recovery trajectories,17,18 while emerging data suggest that chronic stress and psychiatric comorbidities may influence HIV progression and immune markers.19,20
Medication Formulation Changes
Although the patient’s ART regimen remained pharmacologically consistent, the non-clinically indicated switch between manufacturers (from Mylan to Hetero) introduces an additional variable.6 Although both formulations are WHO-prequalified generics, evidence from pharmacovigilance studies suggests that post-market variability can exist between batches or manufacturers.6 In this case, the marginally detectable VL in December (<40 copies/mL) may reflect either a laboratory artifact or a transient viral “blip,” a phenomenon not uncommon even in patients with stable adherence.7,8 Nonetheless, this event underscores the importance of monitoring formulation changes and establishing clear clinical protocols to evaluate potential pharmacological impact.
Systemic and Structural Challenges
The broader structural barriers present in many low- and middle-income countries, including delayed access to confirmatory testing, reliance on external laboratories, and fragmented procurement policies, contribute to diagnostic uncertainty and delays in clinical decision-making.21-23 As shown in this case, laboratory inconsistencies are not merely technical but systemic, reflecting gaps in national quality assurance programs and health policy integration.
This case reinforces the urgent need for harmonized diagnostic standards across all levels of care, integration of external laboratory services within a unified health information system, and expansion of insurance coverage for advanced diagnostics in public sector networks.
Contribution to the Literature and Clinical Practice
Compared to previous reports, this case adds nuanced insights into the interaction between laboratory quality, minor clinical events, and health system structures in shaping the interpretation of CD4 variability. While prior studies have addressed these dimensions in isolation,5-8,13,16 few case-based analyses have illustrated their convergence in a real-world clinical scenario from Latin America.
The report underscores the limitations of relying exclusively on immunological parameters when VL testing is inconsistently accessible, as is the case in many parts of the Global South. In this context, patient-centered clinical judgment, supported by system-level reforms in diagnostic integration, remains critical to ensure accurate and timely decisions in HIV care.
CONCLUSION
This case highlights the complex and interdependent challenges inherent to HIV monitoring in resource-limited settings, where clinical decision-making is often determined as much by systemic constraints as by clinical data. In Ecuador, as in many low- and middle-income countries, essential diagnostic tools such as HIV drug-resistance genotyping, advanced immunological assays, and comprehensive sexually transmitted infection screening remain outside the scope of national reimbursement schemes, despite being considered standard of care under international guidelines. The prohibitive cost of resistance testing, frequently exceeding 2,000 USD per patient, renders such analyses inaccessible to most individuals and healthcare institutions.
As a consequence, national HIV programs are forced to rely on a uniform, first-line, ART initiation policy, with regimen modifications occurring only after immunological or virological failure is detected, rather than being guided by proactive resistance profiling.
This reactive model not only delays optimal clinical management but also increases the likelihood of misinterpreting transient laboratory fluctuations, such as CD4 variability, as indicators of treatment failure. The burden of diagnostic uncertainty thereby shifts from institutional systems to individual clinicians and patients.
This report underscores that CD4 variability must be interpreted within a multidimensional framework encompassing analytical reliability, biological fluctuation, and systemic limitations. Even with strict ART adherence and clinical stability, diagnostic inconsistency can obscure treatment evaluation and undermine trust in laboratory monitoring.
Ultimately, the findings presented here exemplify the broader structural inequities that shape HIV care globally. The inability to fully implement evidence-based diagnostic protocols in under-resourced health systems demands urgent policy reform. Guaranteeing access to high-quality, standardized, and cost-effective diagnostic testing, particularly for VL and resistance genotyping, should no longer be viewed as an aspirational goal but as a fundamental prerequisite for equitable, effective, and sustainable HIV care.







