Abstract
Burkholderia cepacia complex (BCC) infections are rare in immunocompetent individuals. Members of BCC are opportunistic pathogens most often found in patients with cystic fibrosis and chronic granulomatous disease, and immunocompromised patients such as patients with cancer or transplant recipients. BCC can cause chronic pulmonary infection; acute respiratory deterioration, such as cepacia syndrome in patients with cystic fibrosis; sepsis; pneumonia; urinary tract infections; and wound infections in immunocompromised hosts. However, very few cases of septic arthritis and osteomyelitis by this agent have been reported in literature. Here, a 48-year-old male without any clinical evidence of an immunocompromised status presented with pain and fluctuant swelling of multiple large and small joints for 4 weeks. Blood reports revealed anaemia and neutrophilia with raised inflammatory markers including erythrocyte sedimentation rate and C-reactive protein. X-rays of all involved joints showed destruction of articular surfaces of the corresponding bones with evidence of osteomyelitis. An ultrasonography revealed a 32×22 mm collection at the medial aspect of the left knee. Cultures of aspirated fluid from multiple joints yielded heavy growth of BCC. The patient was treated with intravenous ceftazidime and oral trimethoprim/sulfamethoxazole as per the susceptibility report. He also underwent multiple joint aspirations and rehabilitation. BCC-related septic arthritis and osteomyelitis is uncommon in immunocompetent patients, and cases reported in literature are monoarticular. This case highlights that BCC polyarticular septic arthritis with osteomyelitis and soft tissue abscesses is possible in a seemingly immunocompetent adult.
Key Points
1. Burkholderia cepacia complex (BCC) infection mainly involves single joints in elderly patients. The authors report a case of polyarticular septic arthritis in a seemingly immunocompetent 48-year-old adult.2. Appropriate diagnosis is a requisite for successful patient treatment. In this case report, the patient was not diagnosed during the first hospitalisation, when a single joint was involved. This delay led to the involvement of several joints with increased morbidity, financial burden, and prolonged anxiety for the patient and his family.
3. Transmission of BCC infection to the joint is either haematogenous, originating from the respiratory tract where BCC exists as a coloniser, or nosocomial, occurring through contaminated devices or solutions during a prior hospital intervention. The patient gave a negative history for both modes of transmission, but reported walking barefoot. This could have led to a minor foot injury serving as a portal of entry and infection, as BCC can survive for several months in moist soil.
INTRODUCTION
The Burkholderia cepacia complex (BCC) is a group of at least 20 distinct bacterial species within the Burkholderia genus, including Burkholderia cenocepacia, Burkholderia multivorans, Burkholderia vietnamiensis, Burkholderia dolosa, and Burkholderia ambifaria, among others. BCC members are aerobic, gram-negative, motile, catalase-positive, non-lactose fermenting bacilli, formerly known as Pseudomonas cepacia.1 They are found in soil, water, hospital disinfectants, and intravenous fluids. BCC is intrinsically resistant to disinfectants and multiple antimicrobial agents, making outbreaks difficult to control.1 BCC infections are uncommon in immunocompetent hosts. However, they are known to cause respiratory infections (e.g., pneumonia and cepacia syndrome), wound infections, sepsis, and urinary tract infections in immunocompromised hosts, such as patients with cystic fibrosis, chronic granulomatous disease, and cancer, as well as transplant recipients.2 The prevalence of cepacia syndrome is 10% among patients with cystic fibrosis, with a mortality rate of approximately 75%.3
Septic arthritis and osteomyelitis are caused by bacterial and, rarely, fungal pathogens. Prompt diagnosis and intervention are required to prevent important functional complications, post-infectious joint destruction or, more seriously, septicaemia and multiorgan failure.4 Polyarticular septic arthritis (PASA) is a rare entity, represented by the involvement of multiple unrelated joints, which points to a blood-borne mode of spread of the causative organism. Infection can be caused by a variety of bacteria, but the most common pathogens are Staphylococcus aureus, gram-negative bacilli in immunocompromised patients, and Neisseria gonorrhoeae in sexually active individuals.5,6 While BCC members are ubiquitous in nature, they are largely responsible for infections in immunocompromised hosts, or in patients with certain genetic defects.7 Septic arthritis and osteomyelitis due to BCC are highly uncommon in the general population, with very few cases reported in the literature, most of which are monoarticular.8 Reported cases originate from Italy, Lebanon, Canada, Mexico, India, Iran, and the USA.8-12 The role of BCC in infection and pathogenicity is not well described among immunocompetent individuals. This case report highlights that BCC PASA with osteomyelitis and soft tissue abscesses is possible in a seemingly immunocompetent adult.
CASE REPORT
Patient Information
A 48-year-old male was admitted with complaints of multiple painful, swollen joints over the last 4 weeks. He was a car mechanic by profession, living in the suburbs of Kolkata, the capital of West Bengal, a state in eastern India. He gave no history of high-risk sexual behaviour or intravenous drug abuse, and did not have hypertension, diabetes, or other known illnesses. He reported smoking one pack of ‘bidi’ per day and consuming four drinks of country liquor weekly over the past 15 years, which he claimed to have stopped in the last 1–2 years.
Four months prior, the patient had been admitted with pain and swelling in his left knee, but no history of trauma was found. At that time, he had undergone an emergency arthrotomy for suspected left knee septic arthritis, and had been administered oral nonsteroidal anti-inflammatory drugs and opioids for pain relief when experiencing severe, excruciating pain. However, the cultures had then been sterile, and he was discharged on oral antibiotics after 2 weeks.
Over the past 4 weeks, the patient reported developing symmetric inflammatory polyarthritis, predominantly involving the small joints of the hand, wrist, elbow, knee, and ankle, and small joints of the foot, in an additive fashion. The pain restricted his ability to perform self-care activities.
Clinical Findings
Upon admission, the patient was alert, co-operative, and afebrile. His general examination showed no sign of lymphadenopathy, icterus, cyanosis, or oedema, but revealed mild pallor, glossitis, angular cheilitis, poor oral hygiene, and tobacco stains. His BMI was 21 kg/m2, body weight was 50 kg, pulse rate was 110 beats/min, and blood pressure 110/80 mmHg. His systemic examination revealed normal S1 and S2 sounds without murmur, bilateral air entry with clear chest, and normal central nervous system and abdominal findings with no hepatosplenomegaly. A local examination revealed fluctuant swelling over multiple large joints (Figure 1B) with restriction of both active and passive movements. Multiple discharging sinuses were present over the right ankle joint and on the dorsum of the right foot. Routine blood investigations on admission revealed normocytic, normochromic anaemia (haemoglobin: 100 g/L), and neutrophilic leukocytosis (total leukocyte count: 15.7×109/L) with adequate platelet count (320×109/L). Inflammatory markers were raised (erythrocyte sedimentation rate [ESR]: 57 mm/ 1st hour; C-reactive protein [CRP]: 100.32 mg/L). Liver function test and renal function test were normal. Serology for Hepatitis B and C, and HIV were non-reactive. Glycaemic status revealed a fasting plasma glucose of 4.72 mmol/L, postprandial plasma glucose of 5.88 mmol/L, and HbA1c of 43 mmol/mol. Antinuclear antibody and rheumatoid serology tests were also negative. His two paired blood culture samples (24 hours apart) were sterile.
X-rays of all involved joints showed the destruction of articular surfaces of the corresponding bones, and evidence of osteomyelitis (Figure 1A, 1C, 1D). An ultrasonography of the left knee revealed a 32×22 mm sized collection at the medial aspect of the knee and deeper communication with the knee joint.
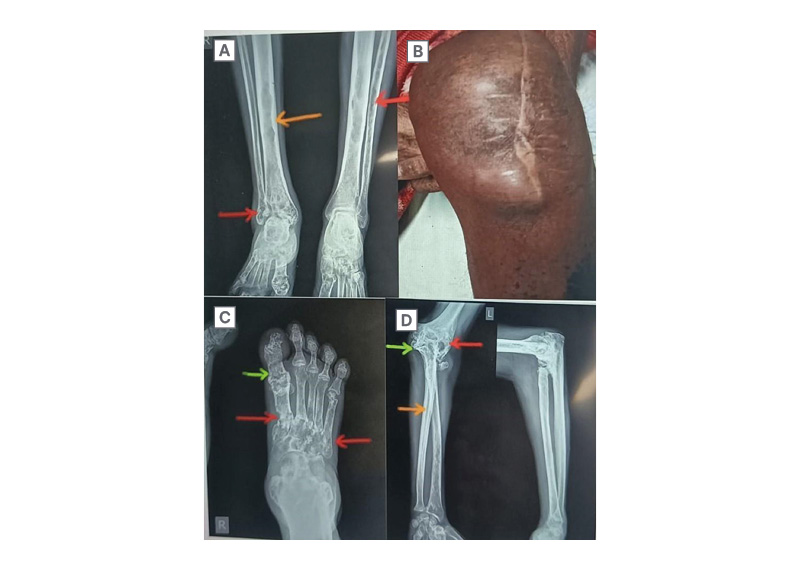
Figure 1: Clinical and X-ray images of various affected sites.
A: X-ray of bilateral leg (anteroposterior view); B: Subcutaneous abscess at left knee joint, post-arthrotomy;
C: X-ray of right foot (anteroposterior view); D: X-ray of left elbow joint (anteroposterior and lateral view).
Red arrows show multiple radiolucent lesions and erosions in the fibula, metatarsals, humerus, and radius; orange arrows show a sclerotic reaction in the shafts of the tibia, fibula, and radial head; green arrows show areas of bony expansion with gross cortical thinning.
The guided aspiration with aseptic measures from the depths of the left knee joint was purulent, with a leukocyte count of 60×109/L (neutrophil: 80%; lymphocyte: 20%) and no evidence of crystals on polarised microscopy. A Gram stain showed a large number of pus cells with long, slender gram-negative bacilli, most of them intracellular (Figure 2). The joint aspirate PCR for Mycobacterium tuberculosis on GeneXpert was negative.
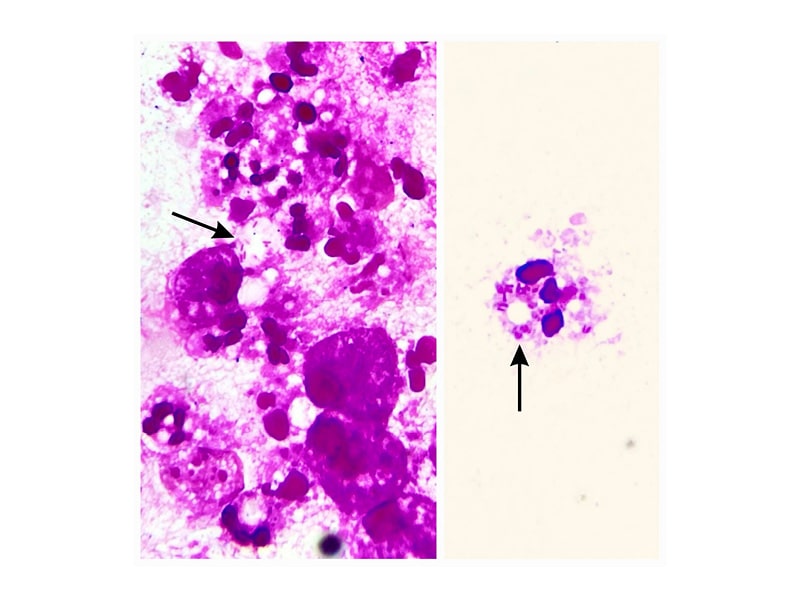
Figure 2: Gram stain from aspirated fluid showing pus cells.
Black arrows show intracellular gram-negative bacilli.
Diagnostic Assessment
Culture showed heavy growth of B. cepacia on two separate occasions from two different sites: the left knee joint and left elbow joint aspirates. Antimicrobial susceptibility was performed by microbroth dilution method by VITEK-2 Compact (bioMérieux, Marcy-l’Étoile, France) as per Clinical Laboratory Standards Institute (CLSI) guidelines. The organism was susceptible to ceftazidime (minimum inhibitory concentration [MIC]: 2 µg/ml), meropenem (MIC: 4 µg/ml) and trimethoprim/sulfamethoxazole (MIC≤ 20 µg/ml).
Therapeutic Intervention
Before culture and susceptibility reports were available, the patient was treated empirically with intravenous meropenem 1 g administered every 8 hours (q8h) for 1 week. Based on the antibiotic susceptibility report, intravenous ceftazidime 2 g q6h and oral trimethoprim/sulfamethoxazole (160 mg/800 mg) 1 tablet q12h were used to treat the patient for the following 8 weeks of hospital stay. MIC of ceftazidime was 2 µg/ml, two dilutions lower than the breakpoint MIC of 8 µg/ml, whereas meropenem MIC was equal to its breakpoint MIC of 4 µg/ml;13 hence, the therapeutic window was larger for ceftazidime compared to meropenem. Furthermore, concentrations of ceftazidime and cotrimoxazole in synovial fluid are typically 80–100 % and 100% of serum levels, respectively, whereas meropenem concentration is 60–100 % of serum levels.14,15 Ceftazidime has moderate soft tissue and bone penetration and good synovial fluid penetration.14,15 In literature, ceftazidime and cotrimoxazole have often been considered the treatment of choice for BCC infections.16 Therefore, instead of increasing meropenem dose to 2 g q8h, the authors decided to switch to a combination of ceftazidime and cotrimoxazole. The patient also underwent multiple joint aspirations and rehabilitation with ankle foot orthosis during this period.
Follow-Up and Outcomes
The patient gradually showed clinical improvement, his total leukocyte count decreased to 9.8×109/L (neutrophil: 70%; lymphocyte: 24%), and inflammatory markers in his blood normalised (CRP: 1.63 mg/L; ESR: 16 mm/1st hour) towards the end of his hospital stay. Upon discharge, he was advised to continue oral trimethoprim/sulfamethoxazole (160 mg/800 mg) twice daily for 6 months, alongside ongoing rehabilitation and monthly follow-ups. After 6 months, his total leukocyte count was 8×109/L (neutrophil: 69%; lymphocyte: 21%), ESR was 14 mm/1st hour, and CRP was 2 mg/L. He has been doing well ever since, without any new joint involvement or soft tissue swelling.
DISCUSSION
To the best of the authors’ knowledge, very few cases of BCC-related bone and joint infections have been reported in the literature. BCC-related infections predominantly affect individuals with compromised immune systems, such as patients with diabetes,11 elderly patients,8,9,11,17,18 patients with malignancies,9 patients with intra-articular steroid injections,19,17 post-transplant recipients, or individuals with a history of substance abuse.8,18 Spontaneous cases of BCC septic arthritis have also been reported, particularly in individuals with underlying conditions like angio-immunoblastic T cell lymphoma,9 or substance abuse.18,8 Prosthetic joint infection due to BCC in a patient with diabetes has also been reported.11 Affected joints vary; the knee and shoulder are the most common sites, with a monoarticular pattern of involvement.
In the authors’ case report, the patient was diagnosed with PASA with osteomyelitis and subcutaneous abscess, despite being clinically immunocompetent. Osteomyelitis by BCC, though rare, has been documented to involve the vertebral column, and the reason for sparing the long bones remains elusive. BCC infection in this young, clinically immunocompetent adult involved several appendicular joints. Several cases of vertebral osteomyelitis have been reported worldwide, some following prior surgeries such as rhinoplasty, cholecystectomy, laminectomy, caesarean section, and bariatric surgery.12,20 Other cases of vertebral osteomyelitis without prior surgery have been reported in patients with cystic fibrosis, intravenous drug abuse, or minor trauma, but sometimes no probable risk factor has been identified.12,18,21 BCC infection largely involves cervical, thoraco-lumbar, or lumbar vertebrae.
Previous Reported Cases of BCC-Related Septic Arthritis
The first case of BCC-related septic arthritis was reported in the USA in a 58-year-old female.17 After receiving multiple intra-articular methylprednisolone injections, she developed septic arthritis of the left ankle joint. She was initially treated with clindamycin, and later switched to gentamicin. After receiving a 33-day course of gentamicin (80 mg intramuscular 3x/day) and undergoing repeated needle aspirations, she recovered successfully without permanent joint damage.
Matteson et al.18 reported right knee joint septic arthritis in the USA in a 72-year-old female with osteoarthritis. BCC was implicated following intra-articular corticosteroid injection.18 The patient received intravenous ceftazidime 2 g 3x/day for 8 weeks. Open drainage and synovectomy were performed, and after completion of treatment, the knee pain resolved.
Sebastian et al.11 from India reported a case of prosthetic joint infection due to BCC in an 85-year-old male with diabetes. The patient presented with left knee septic arthritis following knee replacement surgeries. He received oral cotrimoxazole 500 mg every 12 hours, and ciprofloxacin 750 mg every 24 hours for 2 months, and a revision arthroplasty was performed. There was no recurrence of infection both clinically and radiologically after an 8-month follow-up.
Koo et al.8 reported a case of BCC right knee septic arthritis from Canada, in a 67-year-old male with a medical history of hypertension, gout, osteoarthritis, and intramuscular opioid use.8 He received 3 days of empiric meropenem, and later switched to ceftazidime injections alongside joint irrigation and debridement. After 28 days of antibiotic therapy, his leukocyte counts and CRP levels normalised and his blood culture was sterile. This diagnostic and treatment pathway was very similar to the authors’ patient.
Li et al.18 reported a case of BCC cervical osteomyelitis from the USA in a 68-year-old user of intravenous heroin and methamphetamine.18 He was treated with intravenous Ceftazidime 2 g 3x/day for 6 weeks and prescribed indefinite oral minocycline (100 mg 2x/day). Corpectomy and fusion with allograft placement were performed. At the end of therapy, his inflammatory markers (ESR, CRP) were reduced. Kalash et al.12 reported a case of vertebral osteomyelitis at T12-L1 due to BCC in a 22-year-old female from Iraq post-caesarean section.12 She was initially treated with meropenem for 3 weeks and then switched to oral cotrimoxazole, with a total treatment duration of 8 weeks. After treatment completion, the infection successfully resolved.
Case Analysis
In the authors’ patient, no organism was identified in the initial episode of septic arthritis, possibly due to low bacterial load. However, during the following admission, BCC was isolated from multiple joint fluid aspirates. The patient gave a history of walking barefoot, reporting that most of the time he would not wear footwear. Literature mentions that some BCC genomovars responsible for life-threatening infections in humans have been identified from soil.22,23 The ability of the pathogen to survive in soil is due to its ability to utilise diverse substrates as a carbon source, and tolerate heavy metals present in soil. Isolation of pathogenic genomovars from soil points to human acquisition of infection from sources other than commonly known hospital environments, despite strict infection control measures. Therefore, the authors speculate that their patient may have acquired the infection in this manner, as his respiratory system examination was normal, and there was no history of repeated chest infections or COPD. Immunocompromised status was ruled out by negative serology, euglycemic state, absence of history of intravenous drug abuse and immunosuppressive treatment, negative rheumatoid arthritis factor/antinuclear antibody, and normal serum CD4/CD8 ratio (1.05).
BCC Diagnosis and Treatment
BCC virulence factors confer the ability to form biofilms, which protect from host immunity and perpetuate chronic infection; metabolic adaptation to the tissue microenvironment; capacity for cellular invasion and intracellular survival; evasion of host immunity; and hypermutator phenotype.24 BCC is intrinsically resistant to disinfectants and multiple antimicrobial agents like ampicillin, amoxicillin, ertapenem, polymyxins, aminoglycosides and fosfomycin.1 This is mainly attributed to the efflux pump activity in the bacterial cell wall. Thus, BCC survives and multiplies in aqueous hospital environments, including detergent solutions and intravenous fluids.7 Hence, BCC has been emerging as an important nosocomial pathogen in ICUs, causing pneumonia, wound infection, and catheter-associated infection.25
Diagnosis of BCC is based on blood agar culture and other selective media, or PCR of molecular targets. Recently, matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) is being used to diagnose BCC. Here, the authors relied on microscopy, culture, and identification and sensitivity testing by the VITEK-2 Compact system. The CLSI provides guidelines for antimicrobial susceptibility testing and reporting. Broth microdilution, agar dilution, or E-tests are preferred over disc diffusion methods for BCC susceptibility testing.26 The most active antimicrobial agents against BCC (which warrant their susceptibility testing) are ceftazidime, meropenem, doripenem, minocycline, doxycycline, and trimethoprim/sulfamethoxazole.2 Treatment of septic arthritis involves a combination of surgical intervention and prolonged antibiotic therapy.27 Cases that involved surgical drainage or debridement generally had better outcomes. Antibiotics for septic arthritis are usually given parenterally for 2–4 weeks, later switching to oral antibiotics if the patient is improving.28 However, a prolonged course of antibiotics is required in patients who have infection associated with osteomyelitis or are immunosuppressed. In the case of septic arthritis, the purulent joint fluid should be drained by repeated joint aspiration or arthroscopic/surgical drainage.28 Treatment of osteomyelitis consists of antibiotic therapy and surgical management.29 Surgical exploration and drainage is the mainstay of treatment if there is abscess formation, necrotic or infected bone, failure of medical therapy, or chronic osteomyelitis. Surgical management includes debridement of infected bone and tissues; drainage of abscesses; reconstruction, if necessary, with bone grafts and/or flaps; and in severe or life-threatening infections, amputation may be required.
CONCLUSION
The possibility of BCC PASA, osteomyelitis, and soft tissue abscesses in a seemingly immunocompetent adult is demonstrated by this case. Delays in diagnosis can occur, and the experience can be draining for both patients and medical professionals. Patients can achieve full remission and resume their regular lives following careful evaluation and correct antibiotic administration. Early recognition, targeted antibiotic therapy guided by susceptibility testing, and prompt surgical intervention are critical to ensure favourable outcomes.
Patient Perspective
I had already been admitted to the hospital 4 months prior to this admission and I underwent a surgery in my left knee. At that time, I could neither walk nor carry out self-care activities due to the severe pain. I stayed in the hospital for 2 weeks. However, the swelling reappeared along with pain, 3 months after discharge. This time it affected multiple joints, mainly the left elbow, left knee, and right foot. I had to get admitted once again. I stayed in the hospital for more than 2 months. During my hospital stay, I received injectable medicine, tablets, and my joints were drained multiple times. I also took one tablet twice daily for 6 months after discharge. Now the joint pain and swelling have subsided, and I have returned back to work. I am very thankful to the doctors for bringing me back to normalcy, as I am the sole breadwinner of my family.







