Abstract
Sarcoidosis is a multisystemic disorder of unknown aetiology, characterised by fibrosis and non-necrotising granuloma deposition. Typical presentation includes bilateral hilar adenopathy, skin, eye and articular lesions, pulmonary nodules, serum hypercalcemia, and nephrolithiasis. The authors’ case presented with hypercalcaemia and kidney stones after exogenous vitamin D use, who subsequently was diagnosed with sarcoidosis. Granulomatous diseases like sarcoidosis account for less than 10% of all hypercalcemia cases, and 10–20% of all sarcoidosis patients present with nephrolithiasis. The mechanism of nephrocalcinosis is related to vitamin D and calcium metabolism. Interferon gamma (IF-γ) and IL-2 are released from macrophages activated by T cells. Extrarenal 1-alpha hydroxylation occurs in granulomas, leading to elevation of active 1,25(OH) vitamin D. This causes increased intestinal calcium absorption and osteoclast activity, while tubular reabsorption of calcium decreases due to secondary parathyroid hormone suppression. Vitamin D replacement in this group is a controversial issue. Patients with multisystemic sarcoidosis are usually treated with intravenous methylprednisolone, which increases the risk of bone fractures. However, supplying vitamin D may also worsen hypercalcaemia in patients with sarcoidosis. Previous research indicates vitamin D supplementation may be given in select cases, if the 25-OH levels are low and sarcoidosis is in remission. In conclusion, serum calcium, 24-hour calcium, vitamin D levels, and disease activity should be monitored in patients with sarcoidosis who receive vitamin D supplementation.
Key Points
1. The case describes a patient who presented with severe hypercalcaemia and acute kidney injury. She was on exogenous vitamin D supplements. The patient was diagnosed with sarcoidosis. Haemodialysis was initiated, and steroid therapy was commenced. Nine months into therapy, her calcium and kidney function markers were in their normal range.
2. Sarcoidosis that presents with hypercalcaemia may worsen with Vitamin D supplementation. Therefore, Vitamin D supplements should not be prescribed without a clear deficiency.
3. In some cases, vitamin D may be prescribed to patients with sarcoidosis, especially those receiving steroid treatment. However, 24-hour calcium and parathyroid hormone levels must also be monitored in these patients.
INTRODUCTION
Sarcoidosis is a multisystemic disorder, characterised by fibrosis and non-necrotising granuloma deposition of unknown aetiology. The worldwide incidence is approximately 60/100,000, and this number is estimated to be 4/100,000 in Türkiye.1,2 The female/male ratio of incidence in systemic sarcoidosis is estimated to be 2:1.1,2 The cases are more frequent between 2–6 years of age, half of the cases being above the age of 40 years. Typical findings include bilateral hilar adenopathy; skin, eye, and articular lesions; and pulmonary nodular opacities. Hypercalcaemia and kidney stones related to granulomatosis are frequently encountered. The authors’ case is a sarcoidosis patient who presented with severe hypercalcaemia and acute kidney injury (AKI). She had been using vitamin D3 supplements for 3 years during the COVID-19 pandemic. This case presentation hopes to add to the existing evidence on the effect of vitamin D3 supplementation on sarcoidosis-related hypercalcaemia, as well as arguing against uncontrolled use of vitamin supplements.
CASE PRESENTATION
Initial Presentation
A 63-year-old woman presented to the authors’ nephrology outpatient clinic with nausea, vomiting, and weight loss of 24 kg over 2 years. She was on amlodipin 10 mg/day for essential hypertension, and her blood pressure was within normal range. She had also been using sublingual Vitamin D3-K2 10 puffs/day during and after the COVID-19 pandemic.
Diagnostic Workup
Biochemical studies revealed a creatinine level of 4.7 mg/dL, glomerular filtration rate (GFR) of 10 mL/min/1.73 m2, a serum calcium (Ca) of 14.6 mL/dL, serum phosphorus (PO4) of 3.3 mg/dL, parathyroid hormone (PTH) of 14.59 pg/mL, and 25-hydroxy vitamin D (25(OH)D) level of 52.95 ng/mL. Her initial laboratory studies (Table 1) were significant for AKI and severe hypercalcaemia (Table 1), the calcium level in 24-hour urine was measured as 539 mg/day (reference: 100–320 mg/day), and urine total protein was measured as 479 mg/day.
A urinary system ultrasonography was ordered. Both kidneys were normal in size. In the right kidney, on the level of the right renal pelvis, there was a 13 mm calculous image with a hyperechogenic acoustic shadow. In the right kidney, the renal pelvis revealed Grade 1–2 ectasia, the anteroposterior diameter being 16 mm (Figure 1). In her abdominal CT, Grade 1–2 hydronephrosis in her right kidney was demonstrated. Kidney stones up to 15 mm in her renal pelvis were noted (Figure 1).
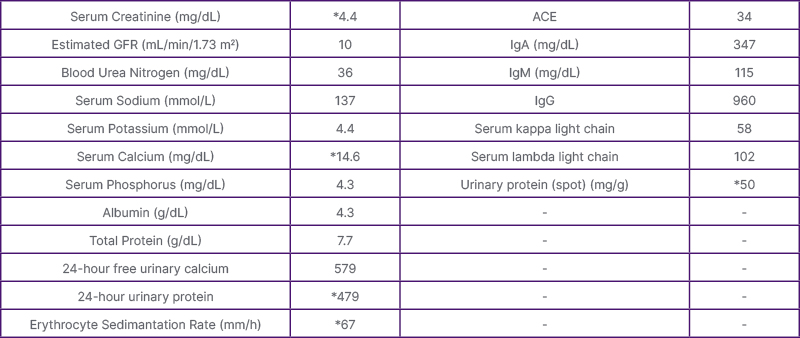
Table 1: The biochemical parameters at presentation. *Parameters outside of the reference range.
ACE: Angiotensin-converting enzyme; GFR: glomerular filtration rate.
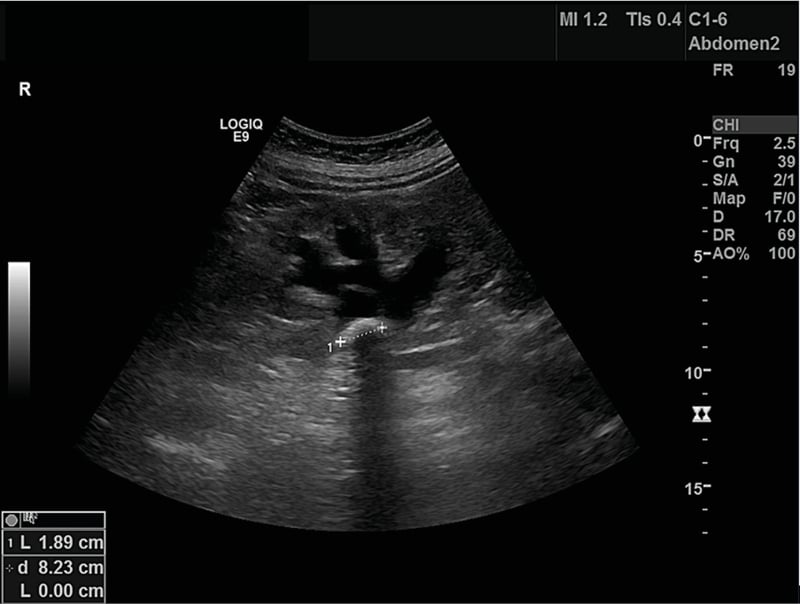
Figure 1: Urinary ultrasonography at presentation.
Hospitalisation
The patient was promptly admitted to the hospital due to acute kidney injury and severe hypercalcaemia. Her Vitamin D supplement was immediately discontinued due to kidney stones and hypercalcaemia, which did not respond to hydration and furosemide therapy. Haemodialysis with a temporary jugular venous catheter was initiated.
Further Studies
Several studies were performed to screen for a malignancy considering high calcium in the setting of low parathyroid hormone. Results for antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), complement, thyroid-stimulating hormone (TSH), and angiotensin-converting enzyme (ACE) were all found to be within normal limits (Table 1). In the serum immune electrophoresis, the IgA was 347 mg/dL, IgM was 115 mg/dL, IgG was 960 mg/dL, serum free Kappa light chain level was 58 mg/L, and serum free Lambda light chain level was 102 mg/L (Figure 2). No plasma cell dyscrasia was found in the bone marrow biopsy. The overall findings were not found to be significant for monoclonal gammopathies.
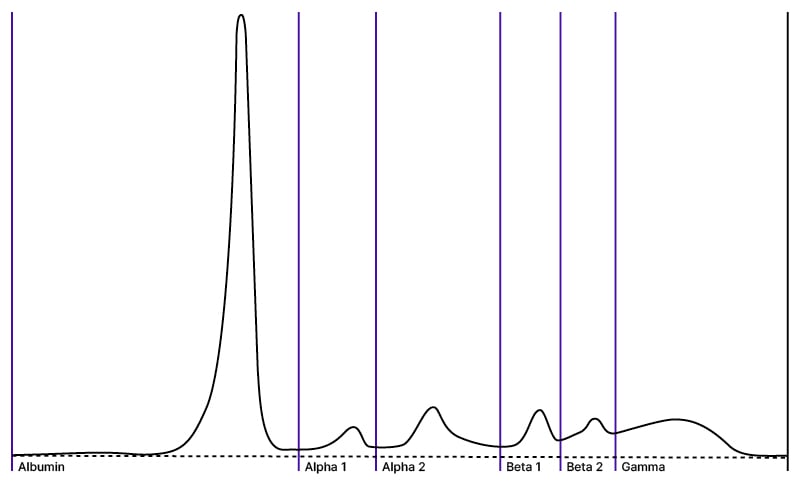
Figure 2: Serum immunoelectrophoresis.
The laboratory testing for multiple myeloma, HIV, and hepatitis all came back negative.
In her PET/CT and posteroanterior chest X-ray, enlarged lymph nodes, the largest being 23×14 mm in the mediastinum, paravascular, bilateral paratracheal, and bilateral hilar spaces were observed (Figure 3). The X-ray also revealed diffuse reticulonodular opacities at this time. A mediastinoscopic lymph node biopsy was performed by thoracic surgery. Diffuse fibrosis with non-necrotising granulomatous inflammation was observed. Occasional CD20+ lymphoid follicules, CD3+ lymphocytes, and CD68+ histiocytes were present; Ki-67 was 5%. No malignant involvement was found, and the findings were reported to be consistent with sarcoidosis.
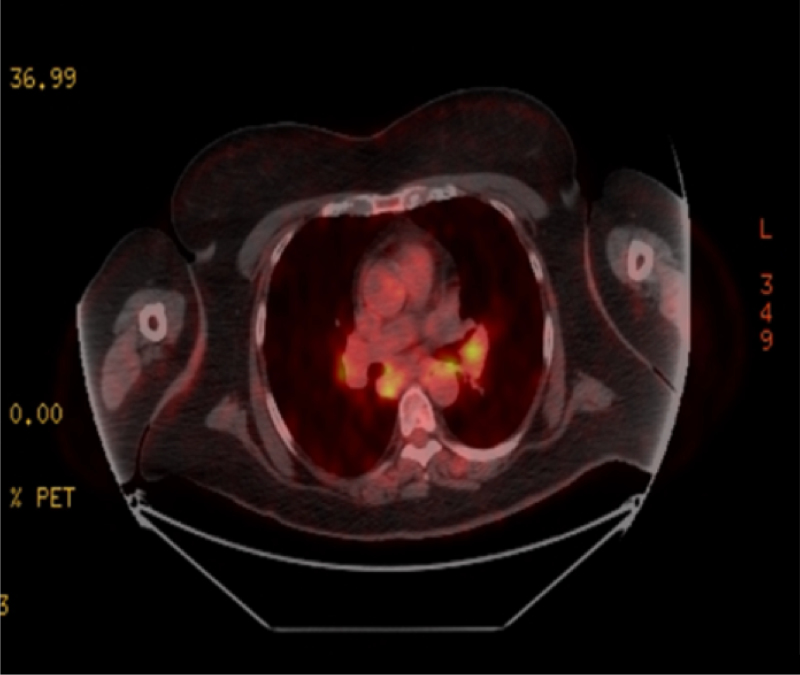
Figure 3: PET/CT before treatment, showcasing bilateral hilar lymphadenopathy.
Treatment and Follow-Up
Methylprednisolone 1 mg/kg was initiated, then gradually tapered down to 5 mg/day over a 1-month period. In the follow-up of 9 months after her treatment was initiated, serum creatinine level was 1.9 mg/dL, e-GFR was 26 mL/min/1.73 m2, BUN was 36 mg/dL, the serum calcium level was 10.4 mg/dL, PO4 level was 3.3 mg/dL, PTH was 55.61 pg/mL, and proteinuria of 30 mg/day (Table 2). The hilar adenopathy was no longer observed on the chest X-ray (Figure 4).
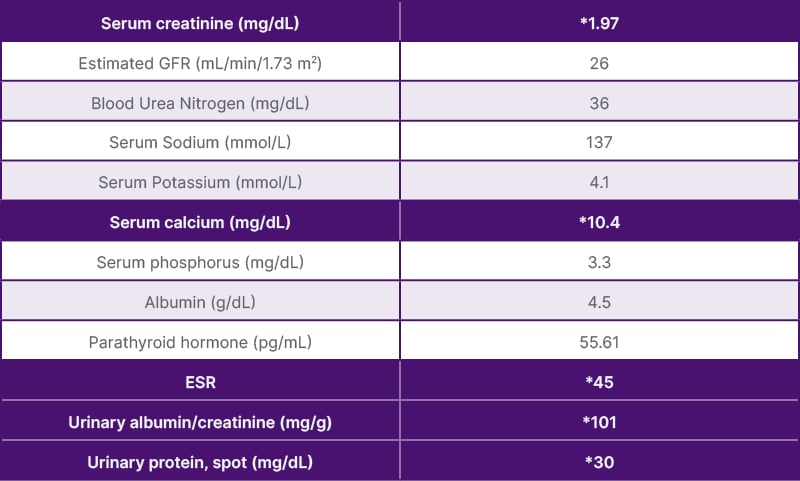
Table 2: Biochemical parameters after 9 months of treatment.
*Values outside of reference range.
ESR: erythrocyte sedimentation rate; GFR: glomerular filtration rate.
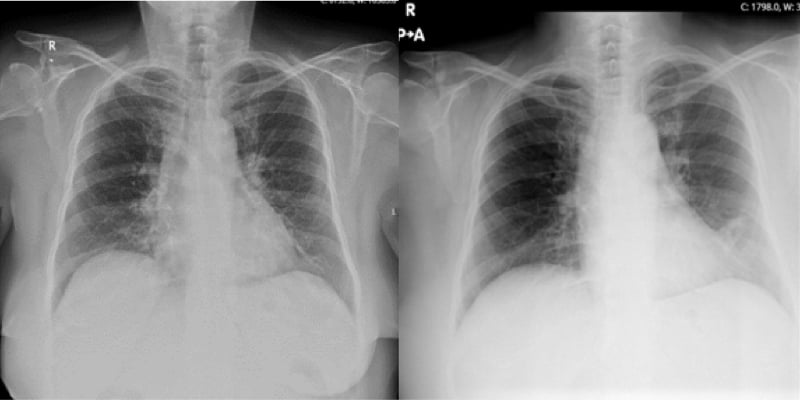
Figure 4: Chest X-Ray before (left) and after (right) treatment.
DISCUSSION
Sarcoidosis was first discovered in 1877 as a granulomatous disorder affecting multiple systems, the cause of which was unknown. The characteristic marking was the presence of noncaseating granulomas, observed most frequently in the lungs and the hilar lymph nodes.
In sarcoidosis, direct renal involvement is seen rarely, but progression can lead to irreversible end-stage renal failure. Since epidemiology data come from small case series with up to 60 patients, the exact incidence of renal sarcoidosis remains unknown.7,11 It has a wide spectrum of presentation, including abnormal calcium metabolism, nephrolithiasis, nephrocalcinosis, and acute tubulointerstitial nephritis with or without granulomas.9,16 Non-granulomatous tubulointerstitial nephritis is the most common histological entity (44%), followed by granulomatous interstitial nephritis (30%), IgA-GN (26%), and nephrocalcinosis (11%).17 Some of the rare manifestations include amyloid A amyloidosis, urinary tract obstruction with renal masses, and glomerular diseases such as membranous nephropathy, IgA nephropathy, minimal change disease, proliferative or crescentic glomerulonephritis, and focal segmental glomerulosclerosis. The presentation of renal sarcoidosis would vary according to the underlying pathology.18-20 Sarcoid-related interstitial nephritis is usually identified as the initial presentation of sarcoidosis and rarely develops among patients who have a longstanding diagnosis of sarcoidosis.13
Kidney stones occur in 10–20% of patients with chronic sarcoidosis. Other studies report an incidence between 6–12%. Kidney stones are thought to peak in Caucasian men between the ages of 40–60 years. Kidney stones are the first finding in 1% of patients with sarcoidosis, and nephrolithiasis is asymptomatic in 2–7% of patients diagnosed with sarcoidosis. However, the risk of progressive kidney damage is increased in sarcoidosis patients with nephrocalcinosis.5 Hypercalcaemia occurs in about 10–20% of patients with sarcoidosis,21,22 while hypercalciuria is more frequent.23-25 The over production of 1,25-(OH)2D depends on the activity of the enzyme 1-alpha hydroxylase, which is expressed in pulmonary alveolar macrophages trapped in granulomatous inflammation and is stimulated by IFN-gamma.22,26 As a result, 1,25-(OH)2D levels in sarcoidosis are directly related to the availability of its substrate, 25-hydroxy vitamin D. 1,25-(OH)2D vitamin D stimulates bone reabsorption by increasing osteoclast activity, augments calcium absorption in the gastrointestinal tract, and increases renal tubular calcium reabsorption.23,27 Since the activity of 1-alpha hydroxylase is refractory to normal negative feedback mechanisms,20 this leads to a progressive increase of calcium in plasma. The consequent suppression of PTH by direct and indirect mechanisms, coupled with an increased renal calcium load, results in the development of hypercalciuria.23,28 Hypercalcaemia causes reduction of GFR and enhances sodium excretion through the inhibition of sodium–potassium ATP-ase, with consequent depletion of total body water, leading to increased bicarbonate reabsorption and metabolic alkalosis.29,30 As a result, calcium reabsorption in the distal nephron increases, aggravating the hypercalcaemia.29 Moreover, hypercalcaemia may be responsible for tubular dysfunction and damage, and for AKI caused by vasoconstriction of afferent arterioles.28,29 In the acute phase, the consequences of hypercalcaemia and hypercalciuria are reversible.27 Nephrocalcinosis, a diffuse calcification of the kidney parenchyma, is the result of prolonged hypercalciuria and is less frequent than nephrolithiasis, the presence of calcium-rich kidney stones.24-32 Renal biopsy can reveal nephrocalcinosis in the initial stages, showing calcium deposits of either calcium phosphate or calcium oxalate.35Severe hypercalcaemia is defined as a calcium level of 14 mg/dL, or 3.5 mmol/L and higher, and an ionised calcium level of 10 mg/dL or 2.5 mmol/L or higher. Primary hyperparathyroidism and malignancy constitute 90% of all hypercalcaemia cases. In the differential diagnosis, granulomatous diseases, including sarcoidosis, tuberculosis, or cat-scratch disease; endocrine disorders like hyperthyroidism; genetic diseases; immobilisation; use of thiazide diuretics; and exogenous use of calcium, vitamin D, or vitamin A supplements should be evaluated. SGLT-2 inhibitors, immune checkpoint inhibitors, discontinuation of RANKL inhibitors such as denosumab, ketogenic diet, SARS-CoV-2 infection, and excessive exercise cause less than 1% of all hypercalcaemia cases. PTH is the first consideration in the differential diagnosis of hypercalcaemia. A high or normal level indicates primary hyperparathyroidism, and a lower level of PTH (<20 pg/mL) indicates a different etiology.36
The mechanism of nephrocalcinosis appears to be linked with vitamin D and calcium metabolism. In granulomatous diseases, interferon-γ (IF-γ) and IL-2 are released from macrophages activated by T cells. Extrarenal 1-alpha hydroxylation occurs in granulomas, leading to elevation of active 1,25(OH)2D. Increased levels of calcitriol cause increased intestinal calcium absorption and osteoclast activity. While calcium filtration increases at the glomerular level, tubular reabsorption of calcium decreases due to PTH suppression.37
Vitamin D is a fat-soluble hormone that has endocrine, paracrine, and autocrine functions. Consumption of vitamin D-supplemented food and drugs has increased significantly since the COVID-19 pandemic. Despite such wide use of artificial vitamin D supplements, the serum level of 25-hydroxyvitamin D does not always reflect the amount of uptake. In contrast to the safe sunlight exposure, prolonged and disproportionate consumption of vitamin D supplements may lead to vitamin D intoxication, even without developing hypervitaminosis D. The reason why vitamin D supplementation is believed to be safe is that even repeated intravenous ingestion of extremely high doses of synthetic vitamin D analogs raise serum vitamin D levels to the toxic range. However, prolonged consumption of vitamin D supplementation may induce hypercalcaemia, hypercalciuria, and hyperphosphatemia, which are considered to be the initial signs of vitamin D intoxication. It is likely that calcium and phosphorus dysregulation, induced by exogenous vitamin D supplementation, leads to tissue and organ damage, even without developing hypervitaminosis.38 Vitamin D replacement remains a controversial issue. In a cohort study conducted with 104 patients, the risk of hypercalcaemia was found to be higher in the patient group not given vitamin D supplementation.39 However, since glucocorticoids are frequently used in patients with sarcoidosis, there is also a risk of skeletal fractures. Administration of exogenous inactive vitamin D supplements is advised to prevent the risk of fractures in patients using glucocorticoids. In a study conducted on 142 patients, 23.5% of the patients had at least one fracture, and the mean age was 51 years. In addition to glucocorticoid exposure, low calcium consumption and 25(OH)D levels higher than 20 ng/mL and lower than 10 ng/mL were found to be risk factors for fractures. Other studies have found that calcium replacement causes a greater increase in serum calcium in patients with sarcoidosis. It was concluded that vitamin D supplementation can be administered if the 25(OH)D levels were low and sarcoidosis was in remission.39
CONCLUSION
The aetiology of the hypercalcaemia in the authors’ patient appears to be the increased rate of conversion of the exogenous inactive 25(OH)D vitamin supplement into the active 1,25(OH)2D, due to increased activity of the 1-alpha-hydroxylase enzyme as a result of granulomatous disease. Moreover, clinicians and patients must be aware that inactive vitamin D serum levels may not always reflect adverse effects of excessive vitamin D use. Therefore, if exogenous vitamin D is supplemented in a patient with sarcoidosis for any reason, disease activity, serum calcium levels, and 24- hour urinary calcium levels need to be closely monitored, as opposed to simply monitoring 25(OH)D levels alone.