Meeting Summary
IgA nephropathy (IgAN) is the most common primary glomerular disease worldwide and a leading cause of kidney failure, especially in younger adults. The approval of nephroprotective and immunosuppressive therapies for IgAN is revolutionising the treatment landscape, which is rapidly evolving beyond traditional standard of care therapies like renin-angiotensin system (RAS) inhibitors. Recent clinical evidence has explored the potential of combined therapies to maximise proteinuria reduction, in alignment with evolving clinical practice guideline recommendations. This symposium delved into the latest advances in IgAN treatment and provided case-based insights and practical strategies, highlighting the critical need to achieve the lowest possible proteinuria levels in patients with IgAN and to optimise individualised patient care.
Early Intervention and Evolving Guidelines
Jürgen Floege, Senior Professor at RWTH Aachen University Hospital, Germany, began with an overview of updated draft Kidney Disease Improving Global Outcomes (KDIGO) guidelines on the management of IgAN.1 IgAN can only be diagnosed with a kidney biopsy, as there are currently no validated serum or urine biomarkers despite extensive research in this area, he explained. To ensure an early diagnosis and prompt treatment, KDIGO, therefore, suggests that kidney biopsy be considered in all adults with proteinuria ≥0.5 g/d (or equivalent) in whom IgAN is a possible diagnosis, and who do not have a contraindication to biopsy.1 It is also recommended to assess for secondary causes. “The big plea of KDIGO is, be more liberal with kidney biopsy,” as treatment options for IgAN are now available, stressed Floege.1 However, he cautioned that biopsy-based MEST-C scores should not be used for therapeutic decisions, only for prognostication purposes.1
“There is no safe level of proteinuria in IgAN,” Floege insisted. Analysis of data from the UK National Registry of Rare Kidney Diseases (RaDaR) cohort showed that, even in patients with proteinuria levels less than 0.44 g/g, most of whom were young adults, nearly 50% progressed to kidney failure or death after 15 years follow-up.2 However, using the IgAN Prediction Tool, which is recommended in guidelines, these patients would be assessed as having only a 10–15% risk of kidney failure/death in the next 18 months, remarked Floege. “Clearly, we need tools with a longer prediction window,” he said, adding “we have to think more than 2 years in advance in patients with IgAN.”
Outlining current treatment options for IgAN, Floege explained that it was important to delineate chronic kidney disease (CKD) therapy from IgA-specific measures. An important message for patients is that they have CKD and will need lifelong treatment, noted Floege, while IgA represents a secondary inflammatory and fibrotic component of the disease that is mediated by the immune system. Lifestyle optimisation, blood pressure (BP) control, and RAS inhibition have been the cornerstone of treatment for IgAN.1 In the past, the approach was to start patients on RAS inhibitors and then wait and see, only adding steroids if there was no improvement over time. However, as Floege explained, “in the course of doing so, estimated glomerular filtration rate (eGFR) was lost, and GFR lost is lost forever.” In what he called a “major step forward,” updated KDIGO guidelines have now proposed full remission as the new treatment aim, defined as proteinuria <0.3 g/day, stable eGFR, and no microhaematuria.1 The recommendation now, therefore, is to add an sodium-glucose co-transporter-2 inhibitor (SGLT2i), and potentially also a low-dose mineralocorticoid antagonist, to the backbone of RAS inhibition.1
Consideration can also be given to replacing the RAS inhibitor with sparsentan, which is a dual angiotensin and endothelin blocker in a single molecule.3 In the PROTECT Phase III active-controlled trial, patients treated with sparsentan showed a nearly 50% reduction in urinary protein-to-creatinine ratio (UPCR) from baseline (the primary endpoint) at Week 36 compared to 15% with irbesartan, and this reduction in proteinuria was sustained over 110 weeks.4,5 Similar results, a rapid fall in proteinuria followed by stabilisation, can also be obtained by adding a pure endothelin antagonist to a patient already on RAS blockade. In the Phase III multinational ALIGN study, the selective endothelin type A receptor antagonist atrasentan led to a 38.0% reduction in UPCR at week 36 versus 3.1% with placebo.6 However, the key outcome in IgAN is eGFR, stressed Floege, and, with data on atrasentan not yet being published, these findings are so far only available for sparsentan. In the PROTECT trial, both sparsentan and irbesartan delayed eGFR loss, but this was increased by approximately 1 mL per 1.73 m2 per year with the dual antagonist sparsentan.4 In terms of adverse events, although systemic BP was not significantly affected, dizziness and hypotension were somewhat more common with sparsentan (15% and 13%, respectively) versus irbesartan (6% and 4%, respectively). Overall, sparsentan was well tolerated, with no cases of drug-induced liver injury and similar peripheral oedema in both groups.4,5
Floege explained that the biggest change in the updated draft KDIGO guidelines was the bidirectionality of treatment, with the recommendation to target IgAN-induced nephron loss and the immune system-mediated disease in parallel.1 He acknowledged this may lead to over-treatment in some patients, but said it was still preferable to the previous approach of slowly uptitrating treatment over a long period of time. Another option for treatment escalation is Nefecon® (Calliditas Therapeutics, Stockholm, Sweden), a targeted-release formulation of the corticosteroid budesonide, with a role in treating the inflammatory component of IgAN.7 In the NeflgArd study, Nefecon 16 mg/day produced a significant decrease in proteinuria over 9 months of treatment.7 Effects on eGFR were also pronounced, with Nefecon saving an average of approximately 5 mL of eGFR versus placebo.7 However, a rebound in proteinuria and resumption of eGFR decline was seen after Nefecon cessation, providing the rationale for an ongoing USA study evaluating half-dose maintenance therapy, continued beyond the initial 9 months of treatment. In terms of safety, Floege stressed that “Nefecon is still a steroid” and, despite a very high first-pass effect in the liver, it is still associated with higher rates of typical steroid-related side effects like acne, headache, and oedema compared to placebo. However, in clinical trials, there were no serious infectious complications or deaths with Nefecon, and no significant increase in de novo diabetes.7
Looking to the future of IgAN therapy, Floege introduced the complement inhibitors as a new therapeutic class targeting both inflammatory and fibrotic disease components, one of which is iptacopan. Iptacopan binds specifically to factor B and inhibits the alternative complement pathway, which plays a key role in the pathogenesis of IgAN.8 In the Phase III APPLAUSE study, iptacopan produced a significant and clinically meaningful 36% reduction in proteinuria versus placebo over 9 months of treatment.8 Floege described the kinetics of this effect as “notable” as the fall in proteinuria with iptacopan occurred slowly, which may equate to true resolution of inflammation. Iptacopan treatment was also well tolerated, with no unexpected safety signals. In particular, Floege pointed out that, even though the APPLAUSE trial was running under pandemic conditions, there was no increase in COVID-19 infections in the iptacopan arm and similar numbers of other infectious events as placebo.8 This study remains ongoing, and eGFR data have not yet been released.
Other therapeutic avenues under active exploration in IgAN include selective A PRoliferation-Inducing Ligand (APRIL) inhibitors, combined APRIL/B cell activating factor (BAFF) inhibitors, and anti-cluster of differentiation (CD) 38 therapy targeting long-lived plasma cells, which are the key producers of IgA.9 All these approaches act to reduce IgA levels, potentially addressing the underlying cause of the disease, Floege surmised.
Case-Based Presentations: Application of Nephroprotective Therapies
From the therapeutic options now available for IgAN, Jörg Latus, Chief Physician in the Department of Nephrology at Robert Bosch Hospital in Stuttgart, Germany, stressed that it was important to focus not only on the different pillars of treatment, but also on timeframes and sequencing. Rather than sequential therapy, he suggested that there may be an argument for treating high-risk patients from the outset with sparsentan, SGLT2is, and Nefecon, using optimised therapy to reduce proteinuria levels. However, Latus also cautioned about the need to stay within the licensed indication of any drug used in IgAN treatment. For initiation of Nefecon or sparsentan, proteinuria levels ≥0.8 g/g and 0.75g/g, respectively, are a label requirement, even in patients previously on RAS inhibition and SGLT2is.10,11
Latus presented two case studies of patients with IgAN from his own nephrology practice treated with combination therapy, and reviewed the clinical evidence supporting each approach. The first was a 46-year-old man diagnosed with IgAN in 2019 with an eGFR of 72 mL/min/1.73m2, proteinuria of 0.8 g/g, and BP of 145/80 mmHg. He was started on RAS inhibition (ramipril 5 mg), followed by the addition of an SGLT2i (dapagliflozin 10 mg). At follow-up, the patient’s eGFR was 46 mL/min/1.73m2, proteinuria 1.1 g/g, and BP 125/80 mm Hg. “First of all, we have to ask if we are satisfied with this level of proteinuria,” Latus remarked, “and the data show that we absolutely should not be, so more therapy is needed.” This patient was, therefore, initiated on sparsentan and, within 4 months, his proteinuria had fallen to 0.3 g/g while eGFR remained stable at 43 mL/min.
Latus then outlined the clinical evidence supporting this chosen treatment approach of initiating sparsentan and cessation of RAS inhibition, plus SGLT2is, given that the pivotal PROTECT study of sparsentan did not include any patients on concomitant SGLT2i therapy.4,5 A recent retrospective, multicentre German study has provided the first real-world evidence of sparsentan’s efficacy in patients already under SGLT2i treatment, he said.12 Latus explained that the 23 patients included in this study were young (median age: 38 years) relative to the PROTECT trial (median age: 47 years) and had well-controlled BP. Prior to the initiation of sparsentan, 100% of patients in the real-world study were on angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB; as per the PROTECT protocol), but a higher proportion were on the maximum labelled dose: 74% versus 65% in PROTECT. Unlike the PROTECT trial, where steroid use in the prior 3 months was an exclusion criterion, 57% of patients in the real-world study had a history of steroid therapy, and 4% were receiving ongoing Nefecon treatment at the time of sparsentan initiation. Baseline renal characteristics indicated a patient population at high risk of end-stage renal disease and with low eGFR (42 mL/min/1.73 m2) compared to PROTECT (57 mL/min/1.73 m2). With the replacement of RAS inhibition with sparsentan, while SGLT2i therapy was continued as background therapy, a significant reduction in UPCR after 14 weeks was achieved, equivalent to a relative reduction in proteinuria up to 62%.12 In terms of remission status, 35% of patients achieved complete remission, and 87% had proteinuria reduction of at least 50% (Figure 1). Latus described these as the most important results “because we want to bring our patients into remission.” Sparsentan also proved well-tolerated in this real-world study with no evidence of serious adverse events, no permanent treatment discontinuations, and no new safety signals compared to the PROTECT trial.12
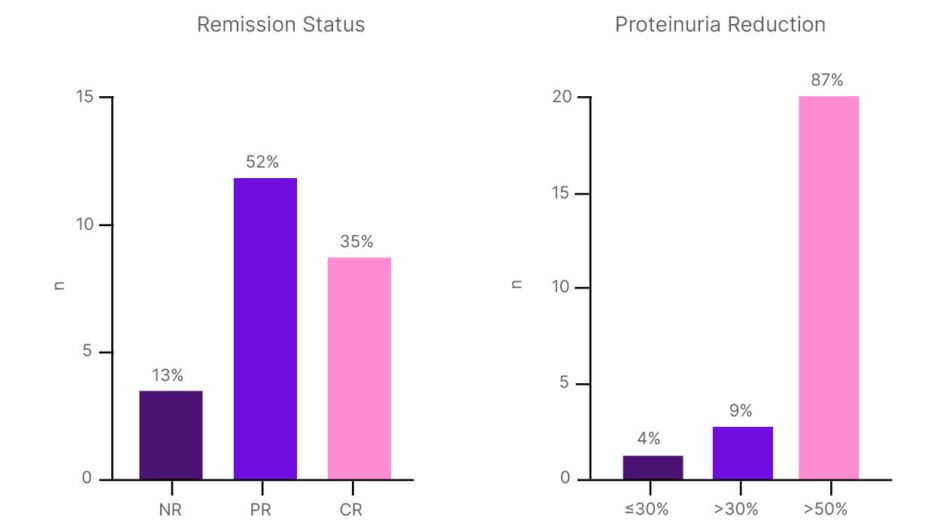
Figure 1: Real-world evidence of sparsentan efficacy in patients on renin angiotensin system inhibitors plus sodium-glucose co-transporter-2 inhibitors.12
CR: complete response; NR: no response; PR: partial response.
Another study evaluating the additive effects of sparsentan on top of SGLT2is is the SPARTACUS trial, which enrolled 48 patients with IgAN. Preliminary analysis of SPARTACUS revealed an additional 40% reduction in the primary endpoint of urine albumin-creatinine ratio (UACR) at Week 24 after initiation of combination therapy.13 In final results from the SPARTACUS trial, presented at ERA 2025, sparsentan added to stable SGLTis demonstrated a 55.8% reduction in UACR at Week 24, with nearly one-third of patients achieving UACR <0.2 g/g.14
The second case study presented was a 29-year-old man diagnosed with IgAN in January 2025, with eGFR of 63 mL/min/1.73 m2, proteinuria of 1.5 g/g, and BP of 128/75 mmHg. Kidney biopsy showed a MEST-C score of M1 E0 S1 T1 C0, although Latus reiterated that kidney biopsy results should not be used as a tool to decide between CKD therapy alone or immunosuppressive treatment. Because this patient was young and at high risk for end-stage renal disease, the decision was made to treat with first-line sparsentan. Evidence for first-line sparsentan in patients with incident IgAN stems from a small open-label, single-arm exploratory trial conducted in 12 patients at five sites in the UK.15,16 Eligible patients with proteinuria >0.5 g/day received 200 mg sparsentan for 14 days, increased to 400 mg thereafter. Interim study analysis showed rapid reductions in proteinuria of approximately 60% by Week 4, which were sustained over the 24 weeks of treatment. Latus pointed out that this reduction in proteinuria surpassed that achievable by initiating first-line RAS inhibition. A decrease in the urinary sCD163-to-creatinine ratio over 24 weeks was also seen, indicating a reduction in inflammation.16
This patient was subsequently started on dapagliflozin, an approach supported by the PROTECT open-label extension study, which showed additive effects in terms of preservation of kidney function and proteinuria reduction in patients treated with sparsentan plus SGLT2i.17 As a final step, Nefecon was also added because, as Latus explained, “it is always important to simultaneously address the CKD and the autoimmune disease.” Evidence from the small cohort of patients in the real-world study of sparsentan plus SGLT2i who had Nefecon before and/or after sparsentan shows that “the three together make sense as combination therapy,” he added.12
Moving forward, Latus suggested that nephrologists should “become more like cardiologists,” optimally deploying approved medications and escalating treatment more quickly in order to, hopefully, consign the use of dialysis for patients with IgAN to history.
Emerging Therapies and Future Directions in IgAN Management
In the drive to develop new IgAN treatment options, a number of novel therapeutic targets are under investigation (Figure 2).9
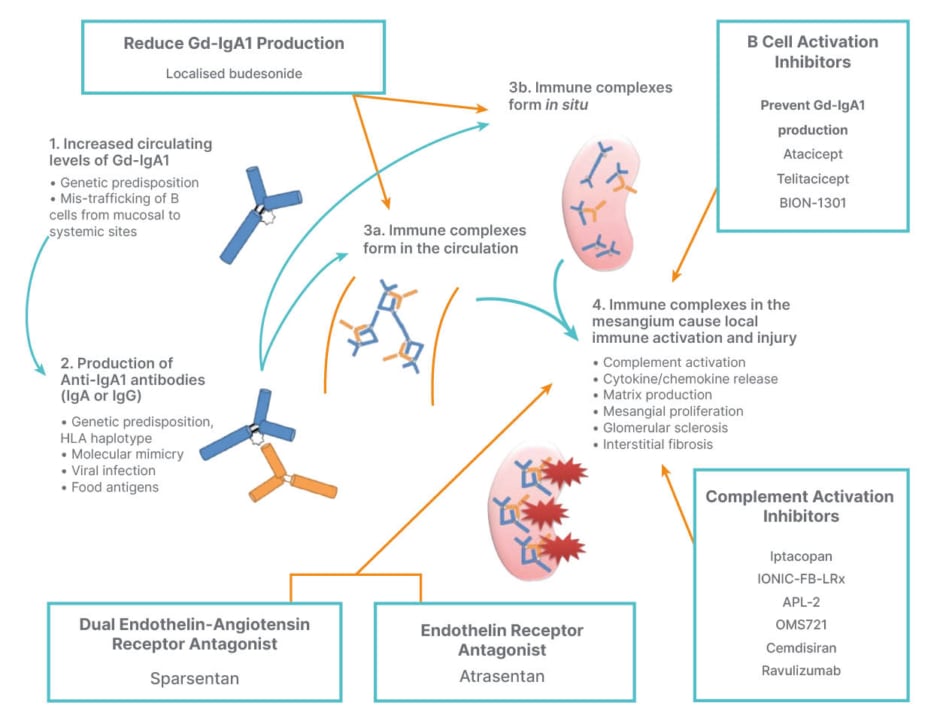
Figure 2: IgA nephropathy novel targets: towards precision medicine.9
GD-IgA1: galactose-deficient IgA1; HLA: human leukocyte antigen.
Of the therapeutic avenues under exploration, Michelle O’Shaughnessy, consultant nephrologist at the University of Galway in Ireland, highlighted BAFF and APRIL inhibitors as “an exciting new group of therapies” currently in late-stage clinical trials. APRIL and BAFF are members of the TNF-α family, which modulate immunoglobulin production, particularly IgA, and have been shown to reduce galactose-deficient IgA1 (GD-IgA1).9 Five of these agents are currently undergoing Phase III trials: the dual BAFF/APRIL inhibitors atacicept, telitacicept, and povetacicpet; and the APRIL inhibitors sibeprenlimab and zigakibart.18-22 Of the APRIL/BAFF inhibitors, O’Shaughnessy described that atacicept had met its primary outcome of significant reduction in proteinuria (46%) compared to placebo (4%) in recently released data from the Phase III ORIGIN trial.23 In the Phase II ORIGIN 2b trial, atacicept showed a sustained reduction in GD-IgA1 (–66%), persistent reduction in proteinuria (–52%), and resolution of haematuria (–75%) over 96 weeks. “Most remarkably, there was complete stablisation of eGFR,” noted O’Shaughnessy, which “if replicated in Phase III studies, will be very exciting.”24
Also under clinical evaluation for IgAN in the fully recruited VISIONARY Phase III trial is the APRIL inhibitor sibeprenlimab. This agent demonstrated a significant proteinuria reduction of 50–60% in Phase II testing, particularly in the 4 and 8 mg/kg dose arms. Notably, although sibeprenlimab was stopped after 12 months, proteinuria reductions were sustained for a further 4 months. The rate of both serious and overall adverse events was similar to placebo.25 Similarly, in prespecified analysis of the Phase III VISIONARY study, presented at ERA 2025, sibeprenlimab achieved a statistically significant 51.2% reduction in proteinuria, as measured by UPCR, at 9 months compared to placebo.26
O’Shaughnessy explained that, in addition to the endothelin receptor antagonists already discussed, a third agent, SC0062, is also undergoing Phase III evaluation, although the study in question is restricted to a Chinese patient population.27 A small trial (ASSIST) looking at the combination of atrasentan and SGLT2is on background RAS inhibition is also underway.28
Complement factor inhibitors help to dampen down inflammation in IgAN and may have positive effects on fibrosis at the tubular interstitial level.9 In addition to iptacopan, which has already received accelerated approval in the USA for the treatment of IgAN, two other complement inhibitors are currently undergoing Phase III investigation: the antisense Factor B inhibitor IONIS-FB-LRX/sefaxersen; and the monoclonal antibody to C5, ravulizumab.29,30 O’Shaughnessy added that three other complement inhibitors have also completed Phase II studies and shown signals for efficacy.31-33 A Phase II trial of the small molecular factor D inhibitor vemircopan was terminated due to lack of efficacy.34 As O’Shaughnessy outlined, results from the SANCTUARY Phase II trial show the “same magnitude of proteinuria reduction with ravulizumab as with other therapies for IgAN.” A 40% reduction in UPCR was achieved at 26 weeks with ravulizumab compared to 11% with placebo, equating to a significant treatment effect (p=0.001).35
In terms of other novel IgAN therapies, the human anti-CD38 antibody felzartamab has demonstrated significant reductions in proteinuria in Phase II trials, with a Phase III study recently initiated in the USA.36 The spleen tyrosine inhibitor fostamatinib has also completed Phase II trials.37
Other potential innovations on the horizon in IgAN include new biomarkers for prognosis and treatment, which could help to guide clinician decision-making around new therapies. These include urinary soluble CD163, usually measured as a ratio to creatinine, which is a marker of macrophage activity.38 Results from Chinese studies have shown that urinary CD163 levels correlate with lesion activity on kidney biopsy and may also predict response to corticosteroid therapy.38 A greater reduction in U-CD163 was seen in patients who received corticosteroids, and this was shown to be associated with better clinical outcomes.38 In terms of histologic findings, O’Shaughnessy explained that high intensity C3 and C4d, as well as a “whole host of other complement proteins of the alternative, lectin, and terminal complement pathways” have been associated with disease severity, worse histological findings, and poorer clinical outcomes in patients with IgAN.39,40 In the future, staining for complement proteins, higher complement levels in the urine, and the presence of complement byproducts in the serum could, therefore, help select patients for treatment with complement inhibitor therapy. In the serum, levels of GdIgA1, anti-Gd-IgA1 antibodies, and IgA1-IgG immune complexes can also be used to predict IgAN severity and disease progression.41
O’Shaughnessy stressed that, as new treatment options for IgAN become available, it is important not to overlook patient comorbidities. Updated KDIGO guidelines include two practice pointers about how to use corticosteroids.1 However, advice on the use of other therapies is “more scant,” she conceded, recommending only that “in all patients in whom treatments that target the production of pathogenic forms of IgA or glomerular inflammation are being considered, a detailed discussion of the risks and benefits of each drug should be undertaken.”1
As we enter this new era of IgAN treatment, O’Shaughnessy highlighted the need for more data on patient groups excluded from prior studies. This includes patients with eGFR <30 mL/min/1.73 m2 and urine protein <0.75 g/day, as well as those with IgA vasculitis, secondary forms of IgAN, paediatric patients, and those with post-transplant recurrence. Some clinical studies are already underway with expanded inclusion criteria and real-world evidence, and observational studies should also help to answer these questions.
Conclusion
It is important to assess patients with suspected IgAN in a timely manner in order to reach a definitive biopsy-based diagnosis. The aim of treatment is to achieve proteinuria below 0.3–0.5 g/day, recognising that some patients will need multi-targeted therapies to reach this goal. Sparsentan has shown efficacy in clinical trials and small real-world studies, even in patients on SGLT2is, while combination therapy with Nefecon and SGLT2is requires further exploration. A raft of novel therapies are also emerging as promising new treatment options for IgAN, and important biomarker research continues.