Interview Summary
Many people living with focal onset seizures (FOS) fail to achieve seizure freedom, even after taking two or more anti-seizure medications (ASM). Others experience adverse ASM side effects, notably psychiatric comorbidities, which have a profound impact on their quality of life (QoL).In the EU, brivaracetam (BRV) is indicated as adjunctive therapy in the treatment of partial-onset seizures with or without secondary generalisation in adults, adolescents, and children from 2 years of age with epilepsy. Designed as an advanced follow-up version of levetiracetam, BRV is thought to have a higher affinity for the synaptic vesicle protein 2A (SV2A) binding site than its predecessor, and does not exhibit modulatory activity at α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, although the effect on clinical safety and efficacy is unknown. RCT evidence has shown the drug to have a favourable efficacy and tolerability profile, but real-world studies are crucial for understanding its performance in broader patient populations and everyday clinical practice.
In this article, Vasilios K. Kimiskidis, Professor of Neurology and Clinical Neurophysiology at the Aristotle University of Thessaloniki in Greece, talks about the BRIVA-Reg study. This prospective, non-interventional, post-marketing study evaluated adjunctive BRV treatment in patients aged ≥4 years with FOS, with or without focal to bilateral tonic-clonic seizures, in the clinical practice setting. Patients were enrolled in six mid-European countries, with an overall study population of 798, and a paediatric population (<18 years) of 56. BRV was prescribed according to routine clinical practice, and in accordance with the approved Summary of Product Characteristics current at the time of enrolment in Europe. Kimiskidis explained that, in the BRIVA-REG study, BRV helped address certain unmet needs in FOS, and talked about the importance of real-world evidence, particularly in diverse and paediatric populations. He also shared his own experience of using BRV in everyday practice.
INTRODUCTION: UNMET NEEDS IN FOCAL ONSET SEIZURES
Epilepsy, a neurological condition characterised by recurrent seizures, affects around 50 million people worldwide.1 FOS, which originate in one hemisphere of the brain, are the most common form of seizure in both adults and children.2
While there have been many advances in recent years, unmet needs remain among people living with focal epilepsy,3 said Kimiskidis. Around one-third of patients with epilepsy experience drug-resistant epilepsy (DRE), meaning they do not achieve freedom from seizures.3 It has been shown that one of the most significant factors affecting QoL of patients with epilepsy is, for instance, psychiatric comorbidity, which can be linked to ASMs.4-6
BRIVARACETAM: AN ADDITIONAL OPTION
BRV emerged as an additional option for some people who experience FOS around 10 years ago, being indicated as an adjunctive therapy for focal seizures with or without secondary generalisation in adults.7,8 Like its predecessor levetiracetam, Kimiskidis explained, BRV is thought to interact with the SV2A protein, which is important for synaptic function.9,10 BRV binds to SV2A with a higher affinity and a greater selectivity than levetiracetam, although the effect on clinical safety and efficacy is unknown.
REAL-WORLD EVIDENCE: BRIVA-REG
While RCTs have established that BRV is an efficacious and well-tolerated drug,7,8 it is essential to understand how it works in the everyday clinical practice indicated population, Kimiskidis explained. This is where Phase IV, post-marketing studies come in. They collect data from everyday clinical practice, containing crucial information about how the drug behaves in the indicated population, which is “our primary interest as physicians,” he added.
Kimiskidis pointed to a clear gap in the BRV real-world evidence base. There have been, he said, a number of studies, but most were focused on Western European countries. “We cannot automatically deduce that, because a drug is well tolerated and efficacious in one country, it should be the same in every other country,” he explained. There is also a lack of data in the 2-year-old and above paediatric population, despite the drug being indicated for this population of patients, he went on.11
The BRIVA-Reg study aimed to help address some of these important gaps. The prospective, non-interventional study included almost 800 people from across Greece, Bulgaria, Romania, Hungary, Czechia, and Poland. Both indicated that paediatric and adult patients were included, people with comorbidities were not excluded, and the drug was prescribed at the discretion of the physician. BRV was prescribed according to routine clinical practice, and in accordance with the approved Summary of Product Characteristics current at the time of enrolment in Europe. Overall, the study offers a clearer picture of how BRV works in the real-world clinical setting, said Kimiskidis.11
As well as measuring efficacy and tolerability, BRIVA-Reg also evaluated QoL. “This was important because, ultimately, we treat patients to improve their QoL,” said Kimiskidis. The Helpilepsy application (Neuroventis, Overijse, Belgium), a digital solution for real-time disease monitoring for patients and physicians, was used for patient questionnaires. Paper questionnaires were not used. However, only around one-third of participants entered their data using the app, and this was listed as a limitation of the study.11
BRIVA-REG: THE RESULTS
Kimiskidis said that about 84% of patients in the overall population had stayed on the drug for 12 months (Figure 1).11 This high retention rate was corroborated by a high responder rate, with 82.4% of the overall population achieving at least a 50% responder rate at 12 months (Figure 2). Kimiskidis highlighted that patients were taking at least one concomitant ASM and had already tried a “high load” of ASMs at baseline.11
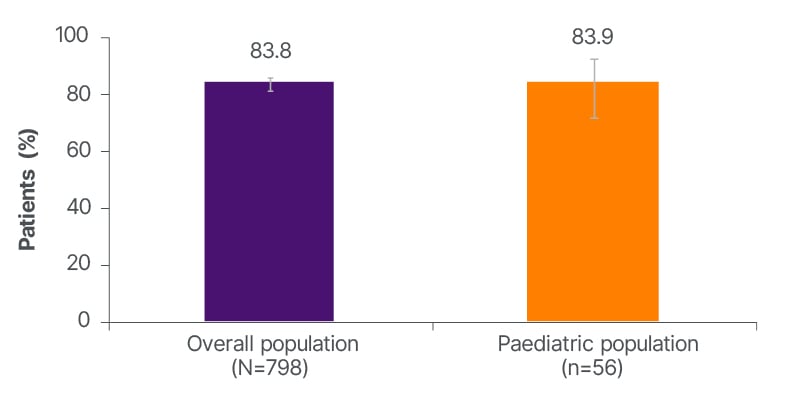
Figure 1: Brivaracetam retention rate at 12 months.
Error bars represent 95% CIs.
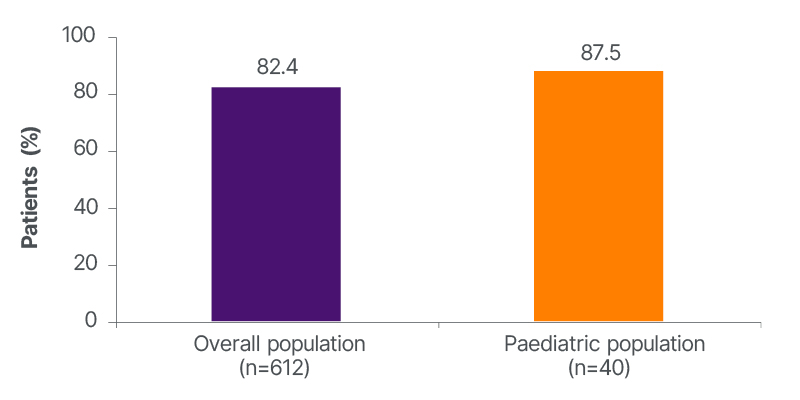
Figure 2: ≥50% responder rate in focal onset seizure frequency at 12 months.
He also highlighted that the results were similar among the overall population (including adults) and indicated paediatric subgroups.11 “It provides the treating physician with evidence that there is a choice he can make when he has a paediatric patient with FOS who does not respond perfectly to the first choice,” he said.
In terms of QoL, in the overall population of 667 people, around 75% of clinicians reported improved Clinical Global Impression of Change (CGIC) at 12 months, compared to baseline (Figure 3).
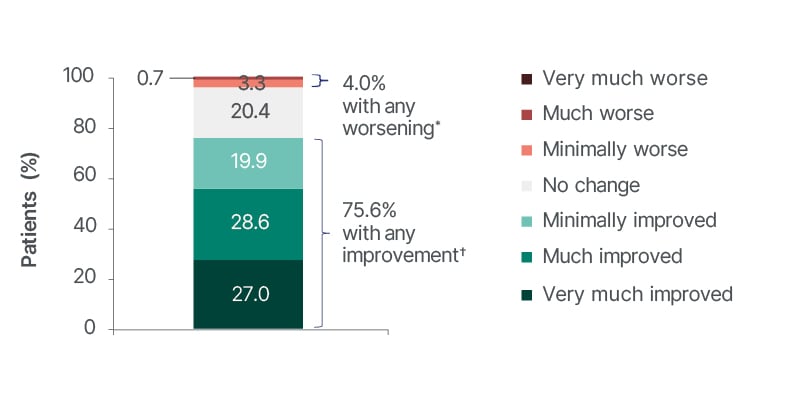
Figure 3: Physician-assessed Clinical Global Impression of Change ratings at 12 months in the overall population.
*Any worsening is the sum of minimally worse and much worse (no patients reported very much worse).
†Any improvement is the sum of minimally improved, much improved, and very much improved.
CGIC is a 7-point categorical rating scale in which the physician is asked to check the number that best describes the patient’s condition over the past 4 weeks compared with baseline. Only the data observed before brivaracetam discontinuation (on brivaracetam) were analysed.
CGIC: Clinical Global Impression of Change.
The study also recorded patient-reported QoL, using different measures for the paediatric and adult cohorts. Median Pediatric Quality of Life Inventory (PedsQL) observed scores improved from 66.33 at baseline to 80.47 at 6 months; however, it was a small sample size (eight at baseline and four at 6 months) as Helpilepsy was the only option to collect Patient Reported Outcome Measures (PROM) data. In adults, the median observed Patient Weighted Quality of Life in Epilepsy Inventory-Form 31 (QOLIE-31P) score was 60.11 at baseline (n=131) and 60.88 (n=59) at 12 months.11
The incidence of treatment-emergent adverse events (TEAE) was comparable to that seen in the randomised clinical trials.9,11 A TEAE was defined as any adverse event that had an onset on or after the date of first BRV administration up to 30 days after BRV discontinuation. In the overall population, 111 (13.9%) people experienced a TEAE, and 63 (7.9%) people experienced a drug-related TEAE.11 There were very few discontinuations following a TEAE (n=34; 4.3%).11
However, there were also some limitations of the study. “I would have liked to see more patients involved from the indicated paediatric population (we had 56), and I would have liked to see more patients engaging with the Helpilepsy digital tool”.
PERSONAL CLINICAL PRACTICE EXPERIENCE
Moving on to talk about his own experience of using BRV in the clinic, Kimiskidis said it seemed to be generally well tolerated. BRV also has a wide variety of pharmacological forms: it can be administered as oral tablets, an oral solution, or intravenously.12
CONCLUSION
BRIVA-Reg provides a window into how BRV works in real indicated patients, in the real world. Although further evidence is needed, particularly in paediatric and international cohorts, current data show the drug’s effectiveness and tolerability. Overall, it highlights BRV’s role as an important step towards helping address some key unmet needs in FOS care, Kimiskidis believes.
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to UCB Pharma Ltd. on 0800 279 3177 or via email at [email protected]. |







