Interviewees: Mark S. Freedman,1-3 Lluís Ramió-Torrentà4
1. Neuroscience Program, Ottawa Hospital Research Institute, Canada
2. Department of Medicine, Neurology, University of Ottawa, Canada
3. Multiple Sclerosis Research Unit, Ottawa Hospital-General Campus, Canada
4. Neurology Department, Dr. Josep Trueta University Hospital, Girona Biomedical Research Institute (IDIBGI), Medical Sciences Department, University of Girona, Spain
Disclosure: Ramió-Torrentà has received compensation for consulting services and speaking honoraria from Biogen, Novartis, Bayer, Merck, Sanofi, Genzyme, Teva Pharmaceutical Industries Ltd, Almirall, and BMS. Freedman has reported receiving research or educational grants from Sanofi-Genzyme Canada; honoraria or consultation fees from Alexion, Atara Biotherapeutics, Bayer Healthcare, Beigene, BMS (Celgene), EMD Inc., Hoffman La-Roche, Janssen (J&J), Merck Serono, Novartis, Pendopharm, and Sanofi-Genzyme; is a member of a company advisory board, board of directors, or other similar group for Alexion, Atara Biotherapeutics, BayerHealthcare, Beigene, BMS (Celgene), Clene Nanomedicine, Hoffman La-Roche, Janssen (J&J), McKesson, Merck Serono, Novartis, and Sanofi-Genzyme; and has participated in a company sponsored speaker’s bureau for Sanofi-Genzyme and EMD Serono.
Acknowledgements: Medical writing was provided by Bronwyn Boyes, London, UK.
Disclaimer: The opinions expressed in this article belong solely to the two named interviewees.
Support: The publication of this interview feature was supported and reviewed by Bristol Myers Squibb.
Citation: EMJ Neurol. 2021;9[Suppl 2]:2-9.
Meeting Summary
This article is based on literature and interviews conducted in early 2021 with two leading experts in neurology, Mark S. Freedman from the University of Ottawa, Canada, and Lluís Ramió-Torrentà from the University of Girona, Spain, who discussed the current approaches and challenges in the assessment of disease activity and progression in relapsing–remitting multiple sclerosis (RRMS).
INTRODUCTION
Multiple sclerosis is a common and chronic neurological disorder that affects over 2.8 million individuals worldwide every year.1 Genetic predispositions, along with environmental factors, play an essential role in the pathogenesis of MS.2 Patients with MS can have various clinical courses, with the most common pattern being RRMS. At diagnosis, approximately 85–90% of patients have RRMS.3-6 RRMS has discrete and clearly defined attacks of new or increasing neurologic symptoms (relapses) followed by periods of partial or full recovery (remissions). During remissions, all symptoms may disappear, or some may continue and become permanent.7
With time, a proportion of patients enter a secondary progressive form (SPMS), with approximately 50% of patients with RRMS transitioning to SPMS within 15 years of the initial MS diagnosis.3-6 The SPMS disease course includes at least an initial relapse, followed by further relapses or gradual neurologic deterioration.8
MS can further be characterised as either active or not active, as well as worsening/with progression or not worsening/without progression. Active disease is defined as with relapses and/or evidence of new MRI activity over a specified period of time. Worsening/with progression is defined as a confirmed increase in disability following a relapse and is evidence of disability accrual over time, irrespective of activity.9,10
In this article, Freedman and Ramió-Torrentà provide their perspectives on this topic of disease activity and progression in patients with RRMS, including an overview of the measurement tools that can be utilised in clinical practice.
MULTIPLE SCLEROSIS BACKGROUND
MS is considered an acquired, immune-mediated inflammatory condition of the central nervous system (CNS), resulting in areas of demyelination, gliosis, and secondary neuronal damage throughout the CNS. The disease course is heterogeneous, characterised by relapses (the acute onset neurological symptoms) and progression (the steady accrual of disability). The underlying pathophysiology is complex, and differences exist in the mechanisms causing key phenotypes.11
Freedman explained that early inflammatory events typically lead to cumulative damage to axons and neurons, which ultimately leads to progression in later phases of the disease. Ramió-Torrentà commented that it is important to note that there are two processes involved: inflammation and demyelination; and neurodegeneration, which can be independent of inflammation.12 Typically, the phenotypes used to characterise MS are RRMS, primary progressive MS (PPMS), and SPMS. Ramió-Torrentà outlined that with the more recent availability of specific therapies to treat RRMS and SPMS, it has become imperative to clearly define these phenotypes. In RRMS, relapses are associated with autoinflammatory processes driven by defects in immune regulation and subsequent migration of multiple effector immune cells across the blood–brain barrier into the CNS. These inflammatory episodes resolve and some lesions remyelinate, but not often successfully or completely and subsequent neuronal degeneration can lead to persistent disability.13,14
Diagnosis is based on neurological signs and symptoms, and evidence of dissemination of CNS lesions.15,16 The McDonald criteria guides the diagnosis of MS by using clinical symptoms, lesions detected by MRI, and laboratory findings.15 Ramió-Torrentà explained that in Europe and Canada the detection of the oligoclonal bands in both cerebrospinal fluid and serum is more frequently utilised for laboratory diagnosis of MS, as compared with the USA. It allows an evaluation of inflammatory processes circumscribed to the CNS and reflects changes in the immunological pattern due to progression.17 In addition, brain volume loss (BVL) has been linked to previous inflammatory activity and is a poor prognostic variable, being associated with cognitive impairment and physical disability progression.18 Clinical trials such as RADIANCE and SUNBEAM, which examined the effect of ozanimod BVL, including thalamic volume and cortical grey matter, highlight that preventing BVL may have important clinical implications affecting treatment decisions.19,20
Freedman believes that disease progression in MS is currently poorly defined, further explaining that some people believe that the substrate for the progression is there from the start but is disguised by the brain and nervous system, which are able to compensate for damage. This compensatory mechanism can happen so quickly that patients do not realise that they have a problem, and the damage may not even show on neurological exam. However, in the later stages, as this inflammation accumulates, the damage starts to exceed the ability of compensatory or regenerative or reparative mechanisms, which all tend to operate better early in the disease. If it outstrips that ability, then you start to see the evidence of the damage (Figure 1).21-23 Freedman explained that the problem is that by the time progression is noticeable (i.e., see patients getting worse and they feel themselves getting worse), the damage has accumulated to the point where it becomes evident. These ‘reserve and repair’ neurological mechanisms explain why MS-related brain damage may go undetected during the early phase of the disease and the delay in diagnosis.
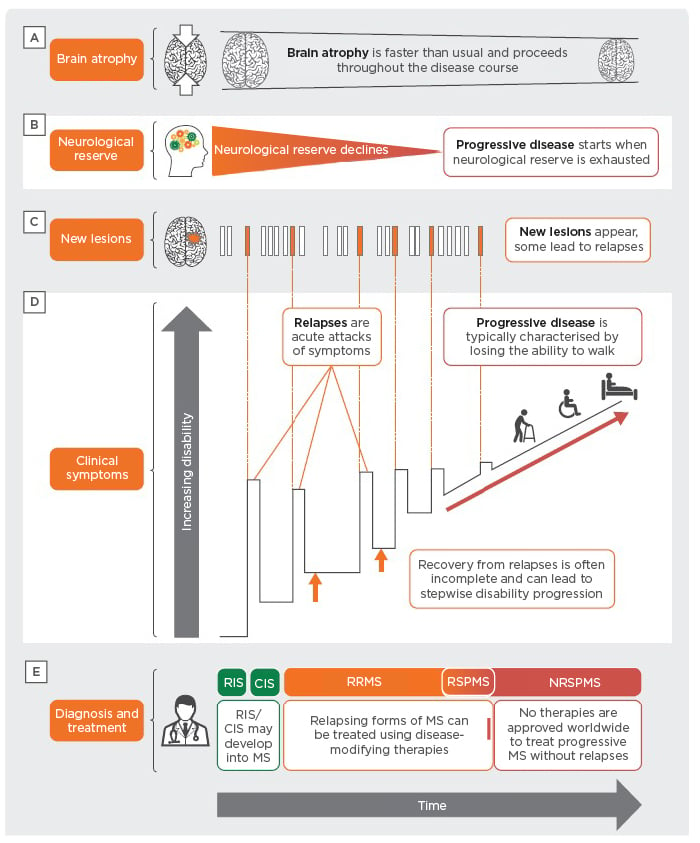
Figure 1: Multiple sclerosis disease pathology.20,22,23,26,30
CIS: clinically isolated syndrome; CNS: central nervous system; MS: multiple sclerosis; NRSPMS: non-relapsing secondary progressive multiple sclerosis; RIS: radiologically isolated syndrome; RRMS: relapsing–remitting multiple sclerosis; RSPMS: relapsing secondary progressive multiple sclerosis.
“The sooner you can diagnose [disease activity in patients with RRMS] correctly, the sooner you can implement effective therapy. And, by the time patients present, even with their first episode, they have had MS for a while. So, it’s important to implement an effective therapy as soon as possible, recognising that already you’ve missed the opportunity, since damage has accumulated in order for that patient to present [with signs and symptoms],” said Freedman.
DISEASE ACTIVITY AND PROGRESSION
MS evolves as a continuum, with an active initial relapsing–remitting course in most patients that, generally, gradually transitions to a phase of progressive accumulation of disability, with or without continued activity (relapses or new inflammatory lesions).24,25 Ramió-Torrentà described the transition from RRMS to SPMS as transitioning from an inflammatory disease to a more neurodegenerative process, independent of inflammatory responses as the key mechanism responsible for disease progression (Figure 1).26
Freedman suggested that the signs and symptoms of permanent neurological disability become evident as repair mechanisms falter and as the functional capacity of the CNS to compensate for these tissue injuries is exhausted.27,28 Studies have reported that the onset of progression is early, with discrete and identifiable signs seen even at a disability status score of 2 or lower.22 In many RRMS patients, silent accrual of disability progression, independent of relapse activity, has also been observed.29 Furthermore, there is evidence that cognitive impairment is present even before the appearance of clinical symptoms of MS. It is therefore important to diagnose MS as early as possible, using all available assessments of cognitive symptoms such as the Symbol Digit Modalities Test (SDMT)30 before the progressive stage of the disease begins.23 Risk factors identified for earlier progression include age at disease onset, sex, number of early relapses, incomplete recovery, and the extent of focal cortical damage at baseline.31 Whole and regional BVL (of the cortex and thalamus), as well as early cognitive impairment are also predictive of poor long-term clinical outcomes in MS.23,29
“The definition of active disease in RRMS is now well-defined, but there is still no consensus on what and how to monitor patients. If we don’t see the patient often enough, then we may miss a relapse or, if we have limited access to MRI (or if we miss the annual MRI), then we may miss radiological activity. As soon as we treat the early signs of active MS, we improve the evolution and the long-term prognosis for the patient,” stated Ramió-Torrentà.
Freedman advocated the Canadian MS Working Group recommendations32 and Ramió-Torrentà mentioned the European Committee of Treatment and Research in Multiple Sclerosis (ECTRIMS), European Academy of Neurology (EAN),33 and Spanish Neurology Society (SEN)34 guidelines to guide assessment of disease activity and progression. Both Freedman and Ramió-Torrentà concurred that the earlier diagnosis of RRMS would enable earlier prescribing of higher efficacy therapies. There may be an optimal window of therapeutic opportunity that, if missed by diagnostic delays, could leave only limited room to affect long-term outcomes in patients with MS. Furthermore, Ramió-Torrentà believes that challenges in diagnosis lies not in the definition but in the measurement tools to utilise.
ASSESSING DISEASE PROGRESSION
The recommended standard tool for demonstrating clinical progression is the neurological examination as documented by the Expanded Disability Status Score (EDSS);35 however, it is widely criticised due to several psychometric limitations.36 Newer MRI techniques have evolved to improve precision and quality, enabling wide use of MRI markers such as new or enlarging T2 lesions and gadolinium enhancing lesions.37,38
Freedman believes that there is a tendency to overuse MRI markers and that confirmation of progression should not be based on MRI alone. Guidelines on MRI in MS are available from the Consortium of MS Centers Task Force and the 2017 McDonald diagnostic criteria.39,40
The quantification of BVL has a good correlate with clinical measures and is one of the most important imaging markers used in progressive MS;35,41 however, both Freedman and Ramió-Torrentà felt that although BVL measurement is important and widely used in clinical trials, it is not currently readily used in clinical practice due to technological and radiology expertise limitations.
Other structural validated biomarkers are under development and validation in progressive MS. Neurofilament light chain is a validated novel marker that can be detected in serum or in cerebrospinal fluid as evidence of neuronal destruction and has good correlation with increased relapses, disability worsening, MRI disease activity, and BVL in MS.42-44 Neurodegeneration markers (e.g., glial fibrillary acid protein) could be a promising complement in the future evaluation of patients with RRMS, as astrogliosis could play a different role in advanced stages of the disease.45 Methods for assessing disease progression are outlined in Table 1. Despite the availability of multiple tools, both Freedman and Ramió-Torrentà spoke about the clinical need for reliable and clinically useful disease assessment and prognostic tools in RRMS.
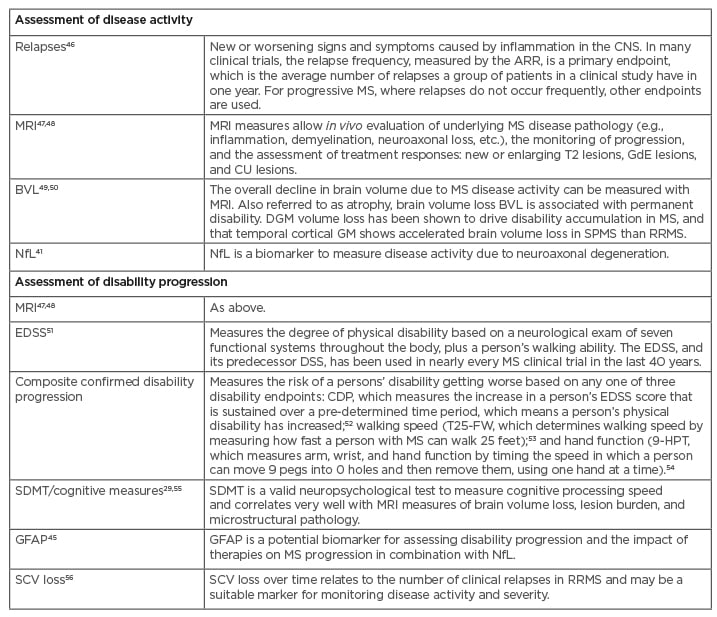
Table 1: Tools to measure disease activity and progression.
9-HPT: 9-Hole Peg Test; AAR: annualised relapse rate; BVL: brain volume loss; CDP: confirmed disability progress; CNS: central nervous system; CU: combined unique; DGM: deep grey matter; DSS: Disability Status Scale; EDSS: Expanded Disability Status Scale; GdE: gadolinium-enhancing; GFAP: glial fibrillary acidic protein; GM: grey matter; MS: multiple sclerosis; NfL: neurofilament light chain; RRMS: relapsing–remitting multiple sclerosis; SCV: spinal cord volume; SDMT: Symbol Digit Modalities Test; SPMS: secondary progressive multiple sclerosis; T25-FW: Timed 25-Foot Walk.
CONCLUSION
The early use of potent disease-modifying therapies (DMTs) may improve the long-term course of MS and reduce permanent neurological damage. The efficacy of DMTs has been shown to significantly reduce the rate of relapses, new or enlarging T2 lesions, gadolinium-enhanced lesions, and BVL in patients with RRMS57 and slow the course of MS progression, particularly when treatment is initiated early.58 A window of opportunity exists in early RRMS to gain maximal benefit from DMTs.30 It is vital, therefore, to monitor disease activity throughout the MS disease course. BVL, which accompanies axonal damage and loss, can be observed early in the MS disease course. Similarly, cognitive impairment is present before the appearance of clinical symptoms of MS.
Delays in the diagnosis of MS and initiation of DMTs allow for the accumulation of axonal damage, progression of BVL, and the development of severe and irreversible neurological disability. Improvements in the capabilities of novel imaging techniques, and increased understanding of the pathobiological mechanisms implicated in the progression of MS to inform the clinical evaluation, have become central in the identification of disability progression in patients with MS. Other tools such as the EDSS and SDMT composite confirmed disability progression are available in neurological practice to document progressive disease.
Despite a growing number of emerging measurements such as BVL and spinal cord volume loss and promising biomarkers (e.g., neurofilament light chain and glial fibrillary acid protein), both Freedman and Ramió-Torrentà spoke about the need for reliable and clinically useful disease assessment and prognostic tools in RRMS. Currently, no simple system of clinical evaluation, cerebral imaging with MRI, or serological test can confirm whether a treatment is achieving the desired outcome and that a patient’s therapy should be stopped or switched. Another key factor is the applicability and useability of the tools in clinical practice. The discovery of simple, reproducible, and inexpensive biomarkers would improve the accuracy and speed of diagnosis of disease activity. It could also enable monitoring of progression, to drive timely therapeutic decisions, and earlier utilisation of DMTs to potentially improve outcomes in patients with RRMS.








