Meeting Summary
A poster reporting real-world data on natalizumab (NTZ; Tysabri®, Biogen, Cambridge, Massachusetts, USA) use in the Swedish Multiple Sclerosis Registry was presented at the Annual Meeting of the European Charcot Foundation (ECF) in Baveno, Italy, in November 2025. The aim of the analysis was to compare the real-world effectiveness of subcutaneously (SC) administered NTZ with that of intravenous (IV) NTZ in NTZ-naïve patients with multiple sclerosis (MS). The study showed that relapse rates were comparable in patients receiving NTZ SC and IV, as were several disability outcomes and imaging findings, supporting NTZ SC as a viable alternative to the IV formulation for patients with MS who are initiating treatment with NTZ.
Introduction
NTZ is a humanised IgG4 antibody that targets α4-integrin expressed on immune cells to block their binding to vascular and mucosal addressin cell adhesion molecules (VCAM and MAdCAM).1 The efficacy of NTZ in relapsing–remitting MS was demonstrated in Phase III trials in which NTZ IV significantly reduced relapse rates, slowed progression of disability, and reduced the formation of new brain lesions.2,3 NTZ was first approved by the EMA for the treatment of relapsing–remitting MS in 2006 as an IV formulation administered by infusion over approximately 1 hour.4 In 2021, an SC formulation was approved by the EMA, providing an alternative option that can be administered in other clinical settings besides infusion centres, with a shorter administration timeframe.5 Approval of the SC formulation was based on studies showing comparable pharmacokinetics/pharmacodynamics, efficacy, and safety to the IV formulation.6,7 As of 31st July 2025, 284,572 people have been treated with NTZ worldwide, representing 1,296,324 person-years, including 36,310 patients receiving NTZ SC over 73,797 person-years (Biogen, data on file). Since May 2025, self-administration or administration by a caregiver is permitted for patients who have tolerated ≥6 doses of NTZ without experiencing hypersensitivity reactions.4 The aim of the current study, presented at the ECF Meeting, was to compare the effectiveness of the two formulations in patients with MS initiating treatment with NTZ for the first time in the real-world clinical setting.8
Retrospective Cohort Analysis of Patients Treated with Natalizumab in the Swedish Multiple Sclerosis Registry
The Swedish Multiple Sclerosis Registry, launched in 2000, holds data on over 24,000 patients with MS in Sweden.8-10 A recent analysis of patients in the registry treated with NTZ between 2006–2024 found that, since the approval of the SC formulation in 2021, 61% of patients initiating NTZ treatment have started on NTZ SC from the outset of treatment. The other 39% started on NTZ IV, with 12% of those patients subsequently switching to the SC formulation.11
Glaser et al.8 conducted a retrospective cohort study in patients in the Swedish Multiple Sclerosis Registry who initiated treatment with NTZ 300 mg IV or SC between January 2019–November 2024. A total of 1,532 patients initiated NTZ during this period, of whom 1,041 met all criteria for inclusion in the current analysis: no prior NTZ treatment; at least 6 months follow-up from treatment initiation; and no switch between IV and SC formulations during follow-up. The analysis population comprised 378 patients on NTZ SC and a propensity score (PS)-weighted cohort of 663 patients on NTZ IV, with similar demographic and disease characteristics (65% and 68% female in SC and IV cohorts, respectively; mean age 37 versus 36 years; median disease duration approximately 6 months versus 8 months; a mean of 0.23 versus 0.34 relapses in the 12 months prior to treatment initiation; 24% versus 28% had received prior treatment with disease-modifying therapies).8
Efficacy of Subcutaneous and Intravenous Natalizumab
The primary analysis outcome of time-to-first-relapse showed no significant difference between SC and IV cohorts (hazard ratio [HR]: 0.75; 95% CI: 0.37–1.52; p=0.429; Figure 1). Annualised relapse rates (ARR) were also similarly low in both SC and IV cohorts (0.0185 versus 0.0197; p=0.856; Figure 2A). Within the SC cohort, the on-treatment ARR was compared with ARR in the 12 months prior to treatment initiation; ARR was significantly reduced, from 0.9410 to 0.0185 (p<0.0001), following initiation of NTZ SC (Figure 2B).8
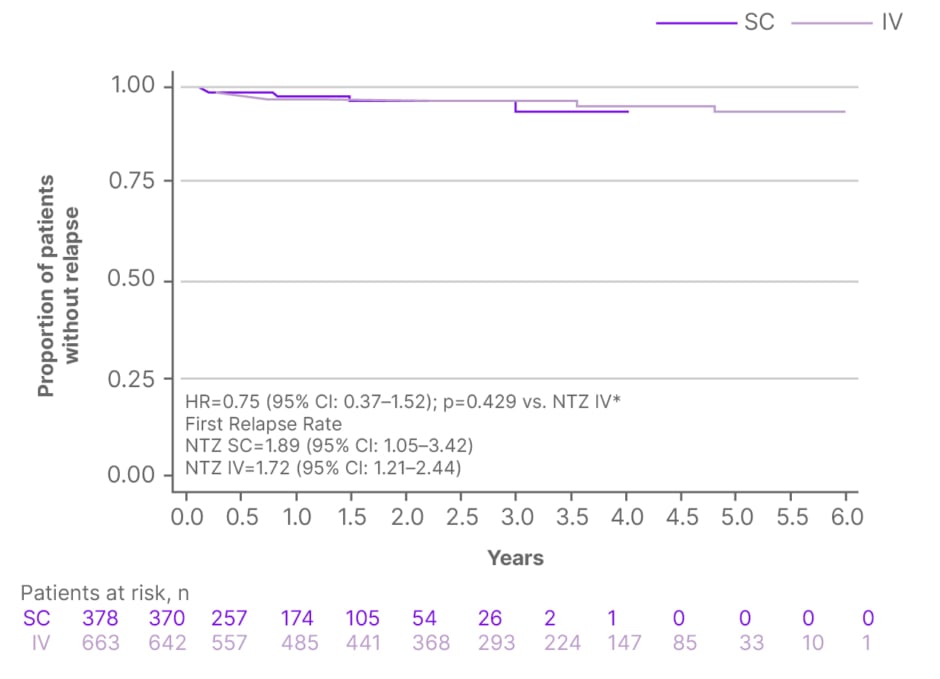
Figure 1: Time to first relapse in patients treated with subcutaneous or intravenous natalizumab.8
*Cox proportional hazards regression analysis.
HR: hazard ratio; IV: intravenous; NTZ: natalizumab; SC: subcutaneous; vs.: versus.
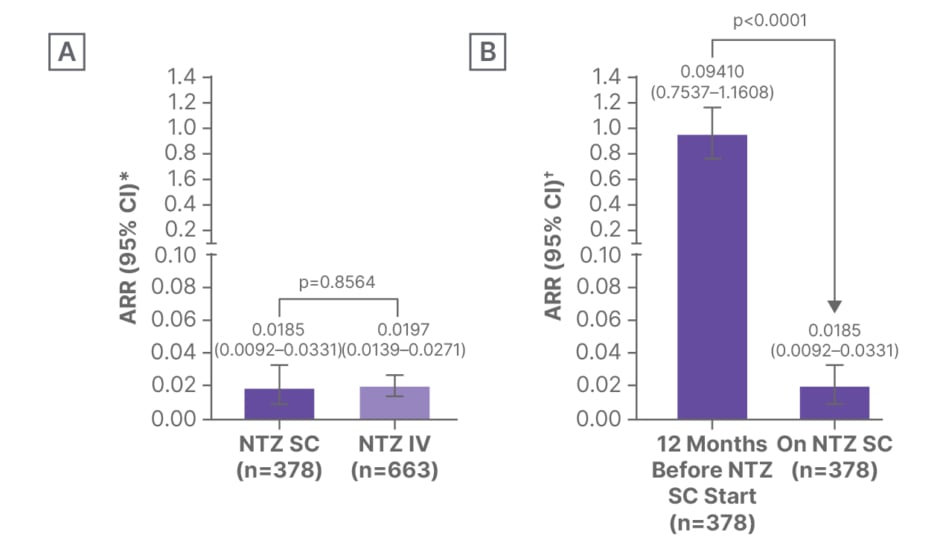
Figure 2: Annualised relapse rates in patients in the Swedish Multiple Sclerosis Registry who were treated with natalizumab.8
*Exact Poisson method with log of follow-up time (year) since NTZ initiation adjusted as offset in the model.
?Negative binomial regression.
A) NTZ SC versus NTZ IV cohorts. B) Before versus during NTZ treatment in the SC cohort.
ARR: annualised relapse rate; IV: intravenous; NTZ: natalizumab; SC: subcutaneous.
The SC and IV formulations also had similar effects on disability outcomes, assessed based on confirmed disability worsening (weighted HR: 0.34; 95% CI: 0.04–3.15; p=0.342), confirmed disability improvement (weighted HR: 0.56; 95% CI: 0.16–1.98; p=0.365), and mean change from baseline on the Expanded Disability Status Scale (EDSS; SC cohort β coefficient: 0.08; 95% CI: −0.27–0.42; p=0.655).8
Appearance of new gadolinium-enhancing or new/newly enlarging T2 lesions was assessed over 4 years among patients with lesion data from ≥1 MRI scan. The rate of new lesions declined in both NTZ SC and IV cohorts, with no statistically significant differences in any time period (Figure 3).8
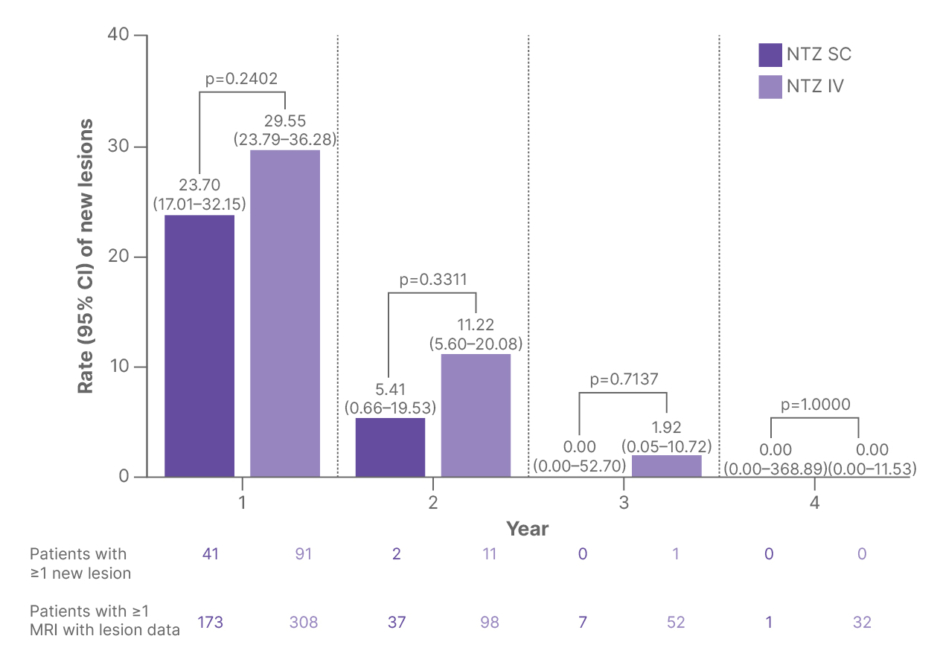
Figure 3: Rates of new gadolinium-enhancing or new/newly enlarging T2 lesions over time in patients treated with subcutaneous or intravenous natalizumab.8
IV: intravenous; NTZ: natalizumab; SC: subcutaneous.
A sensitivity analysis was conducted in patients who initiated NTZ in 2021 or later (after approval of NTZ SC), and showed similar outcomes between the NTZ SC (n=377) and NTZ IV (n=394) cohorts for time-to-first-relapse (HR: 0.96; 95% CI: 0.43–2.15; p=0.915) and ARR (0.0186 [95% CI: 0.0093–0.0333] versus 0.0179 [0.0103–0.0291]; p=0.9235). A further sensitivity analysis in PS-matched cohorts (n=206 patients in each cohort, matched 1:1 for PS-weighted baseline characteristics) also showed similar results to the main analysis for all outcomes except change in EDSS (SC cohort β coefficient: 0.49; 95% CI: 0.11–0.86; p=0.012).8
Conclusion
This retrospective analysis of patients in the Swedish Multiple Sclerosis Registry initiating treatment with NTZ showed that the real-world effectiveness of NTZ SC is comparable to that of NTZ IV in NTZ-naïve patients. This finding, along with previous studies of NTZ SC and IV formulations,6,7,12-15 supports NTZ SC as a viable treatment alternative for patients with MS who are initiating treatment with NTZ for the first time.
| Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store (UK). Adverse events should also be reported to Biogen at 0800 008 7401 (UK) or at [email protected]. |
©️2025 Biogen
Biogen-277659
Date of Preparation: December 2025







