Meeting Summary
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a rare immune-mediated disorder that affects sensory function and muscle strength. This article summarises selected data from poster and oral presentations on CIDP and the neonatal Fc receptor blocker, efgartigimod, at the European Academy of Neurology (EAN) Congress held in June 2025 in Helsinki, Finland.
In the pivotal ADHERE trial, subcutaneous (SC) efgartigimod PH20 demonstrated clinical efficacy in the treatment of patients with CIDP, reducing relapse risk and improving disability scores. Data from post hoc analysis of the ADHERE trial exploring the effect of efgartigimod on lower limb function were presented by Simon Rinaldi from the University of Oxford, UK. Results showed that selective reduction of IgG with efgartigimod PH20 SC treatment improved lower limb function in patients with CIDP. Notably, over 20% of patients (33/144) who required support to walk at run-in baseline (the moment when patients had to stop their prior treatment) could walk outdoors independently after efgartigimod treatment in the open-label stage of ADHERE (Stage A), and over a third (9/25) who were previously wheelchair-bound were able to walk outdoors with support.
Looking at CIDP through a broad lens, Sandra Paci from argenx in Ghent, Belgium, presented results from a real-world multinational survey that compared patients’ satisfaction with treatment across five European countries. Although some variability in treatment satisfaction was observed across the different countries, a clear association between disability level, fatigue, and satisfaction level was evident. The higher the level of disability and fatigue, the lower the probability of patients being satisfied with their treatment.
Introduction
CIDP is a severe, immune-mediated disease characterised by progressive or relapsing weakness in the proximal and/or distal muscles and sensory disturbances, leading to disability.1,2 IgG autoantibodies have been implicated as key mediators in the underlying pathophysiology of CIDP as part of a humoral-mediated immunobiological process.3,4
Efgartigimod is a human IgG1 antibody fragment that blocks the neonatal Fc receptor and results in targeted reduction of IgG levels, without affecting other immunoglobulins or reducing albumin.4-8 Coformulation of efgartigimod with recombinant human hyaluronidase (also known as PH20) allows for rapid administration of larger volumes as a single SC injection.8,9 Efgartigimod PH20 is currently approved in several countries, including the USA and Japan, for the treatment of adult patients with CIDP. As of 19th June 2025, the European Commission has also approved efgartigimod PH20 as a monotherapy for the treatment of adult patients with progressive or relapsing active CIDP after prior treatment with corticosteroids or immunoglobulins.8-10
Efgartigimod PH20 SC was evaluated clinically in the Phase II, double-blind, placebo-controlled ADHERE study, the largest randomised trial in CIDP to date.4 In this pivotal study, treatment with efgartigimod PH20 SC was associated with early onset of clinically meaningful improvements in disability scores and grip strength in patients with CIDP, and significantly reduced the risk of relapse compared to placebo in those who responded to treatment.4
Effect of Efgartigimod PH20 SC on Lower Limb Function in Chronic Inflammatory Demyelinating Polyradiculoneuropathy in ADHERE
Post hoc analysis of the ADHERE study was carried out specifically to evaluate the effect of efgartigimod PH20 SC on lower limb function.11 The trial itself involved a run-in period (≤12 weeks; during which those on traditional CIDP therapy stopped their treatments to confirm active disease), followed by the open-label Stage A (≤12 weeks) in which all participants received 1,000 mg weekly efgartigimod PH20 SC.4 Responders were then randomised 1:1 to efgartigimod PH20 SC or placebo for ≤48 weeks in the double-blind Stage B.4 Thereafter, participants could continue to receive efgartigimod PH20 SC in the ADHERE+ open-label extension study which evaluated long-term safety and efficacy up to 2 years.
Outcomes assessed in the ADHERE trial included changes in adjusted Inflammatory Neuropathy Cause and Treatment (aINCAT) leg disability score, selected Inflammatory Rasch-built Overall Disability Scale (I-RODS) individual items, and Timed Up and Go (TUG) test. In total, 322 participants entered Stage A, of whom 221 with evidence of clinical improvement (responders) were randomised and treated in Stage B, where 111 patients received efgartigimod PH20 SC and 110 received placebo.
Post hoc analysis showed that treatment with efgartigimod PH20 SC improved the time taken for patients to complete the TUG test by Stage A last assessment. In Stage A responders, TUG time was reduced by 3.6 seconds with efgartigimod PH20 SC treatment compared with run-in baseline. The TUG test completion time continued to improve in these participants, reaching a difference of 5.0 seconds at Week 36 of the ADHERE+ trial.11
Efgartigimod PH20 SC also improved lower limb function, as demonstrated by patients’ ability to perform selected I-RODS items such as walking up one flight of stairs, walking while avoiding obstacles, walking outdoors, and running. The proportion of participants who could easily perform these lower limb-dependent tasks increased during Stage A following efgartigimod PH20 SC treatment. (Figure 1A) Maintenance of, or further improvement in, efficacy was observed for patients randomised to efgartigimod PH20 SC during Stage B, while a loss of efficacy was observed in those who received placebo. (Figure 1B)11
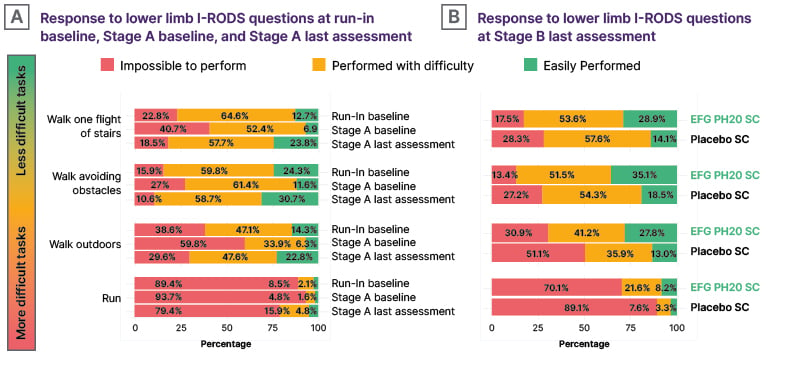
Figure 1: Improvement in ability to perform selected Inflammatory Rasch-built Overall Disability Scale items with efgartigimod PH20 subcutaneous treatment.11
The denominator for the percentage calculations in A) is based on all patients enrolled in Stage A with a run-in phase (N=286). The denominator for the percentage calculations in B) is based on all patients randomised in Stage B with a run-in phase (N=191).
EFG PH20 SC: subcutaneous efgartigimod PH20; I-RODS: Inflammatory Rasch-built Overall Disability Scale; SC: subcutaneous.
Finally, some participants experienced substantial improvements in their INCAT leg disability scores with efgartigimod PH20 treatment. By Stage A last assessment, 23% of participants who had required support to walk outdoors (INCAT scores: 2–3) at run-in baseline were able to walk outdoors independently. (Figure 2A) Additionally, 36% of participants who were wheelchair bound with INCAT leg scores of ≥4 at run-in baseline could walk outdoors with or without support after efgartigimod PH20 SC treatment in Stage A. (Figure 2B) However it should be noted that, as sample sizes in this analysis were small, further studies using real-world data are required to provide further validation of these findings.11
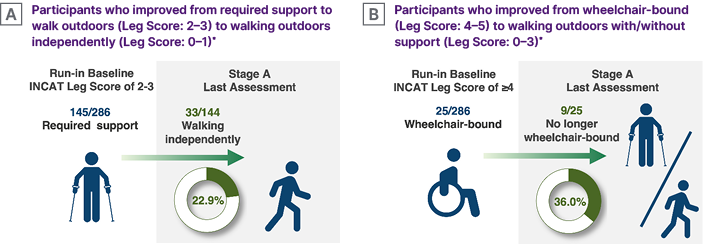
Figure 2: Improvements in Inflammatory Neuropathy Cause and Treatment Leg Disability Score with efgartigimod PH20 subcutaneous treatment.11
A) This is based on all patients enrolled in Stage A with a run-in phase and with an INCAT lower limb score of 2–3 at run-in baseline (N=145). B) This is based on all patients enrolled in Stage A with a run-in phase and with an INCAT lower limb score of 4–5 at run-in baseline (N=25).
*After having undergone mandated CIDP deterioration during the run-in period.
INCAT: Inflammatory Neuropathy Cause and Treatment.
In conclusion, these post hoc analyses show that selective reduction of IgG with efgartigimod PH20 SC treatment resulted in improved lower limb function in participants with CIDP.11
A Comparison of Treatment Satisfaction in CIDP across Five Countries: Results from a Real-World Multinational Survey
Traditional treatments for CIDP include corticosteroids (CS), intravenous immunoglobulin (IVIG) and plasmapheresis (PLEX). These therapies are associated with a high treatment burden due to issues such as long-duration infusions and poor tolerability.12-14 Traditional treatment options for CIDP are also hampered by limited efficacy and lack of clinical response, meaning patients may experience residual neurological impairment and disability.12-14
Due to limited research in this area, little is currently known about the satisfaction of patients with CIDP with available treatment options. To address these gaps in understanding, a real-world study with neurologists and patients with CIDP was carried out in Spain, France, Germany, and Italy. Key aims were to compare patients’ satisfaction with CIDP treatment among European countries, evaluate the association between treatment satisfaction and disability and fatigue, and assess the concordance between patient and neurologist satisfaction.15
Data were collected in the four European countries via cross-sectional physician and patient surveys carried out as part of Adelphi’s CIDP Disease Specific Programme™, which took place between September 2022–April 2023. Neurologists completed electronic record forms, including a question about their satisfaction with their patient’s treatment. Patients filled in a self-completion form, including questions about their overall treatment satisfaction. These patient and physician data sources were then linked, giving a total sample size of 199 patients. Overall, 57% of patients included in the analysis were male and mean age was 52.4 years. Since both forms had different wording for satisfaction, the two highest options were grouped and this was considered the proportion of patients or neurologists who were satisfied. Therefore, respondents giving one of the two highest ratings on the patient form (somewhat/very satisfied) or the neurologist form (satisfied/completely satisfied) were ranked as ‘satisfied’. Two measures related to disease severity were also assessed: disability, measured using the INCAT score; and fatigue measured by the Functional Assessment of Chronic Illness Therapy (FACIT) score.15
Country comparisons showed that the greatest satisfaction with CIDP treatment was in Germany, where 78% of patients reported being satisfied. Germany also had the highest proportion of patients with CIDP on treatment (94%) when compared to Italy (79%), Spain (75%), and France (63%). The lowest satisfaction was observed in France, where only 56% of patients reported being satisfied with their current CIDP treatment. However, it should be noted that sample sizes per country were low, and there were differences in the distribution of disease severity.15
Results from this real-world multinational survey also showed a strong association between treatment satisfaction and both disability and fatigue. The higher the patients’ level of disability and fatigue, the lower the probability of their being satisfied with their CIDP treatment. (Figure 3) Overall, only a third of patients with severe disability were satisfied with their current treatment, as were less than 20% with severe fatigue.15
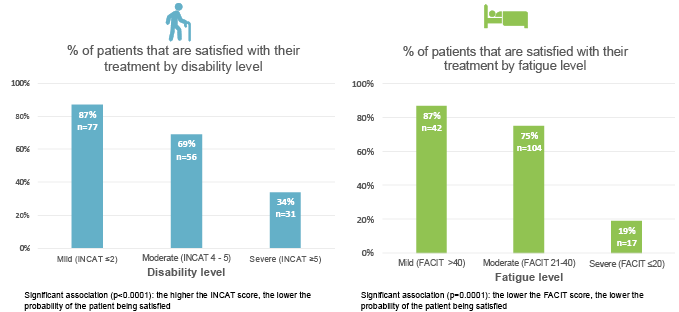
Figure 3: Association between patient satisfaction with current treatments and disability/fatigue.15
Current treatment refers to IVIG, CS, or PLEX.
CS: corticosteroids; FACIT: Functional Assessment of Chronic Illness Therapy; INCAT: Inflammatory Neuropathy Cause and Treatment; IVIG: intravenous immunoglobulin; PLEX: plasmapheresis.
In terms of concordance between patient and physician satisfaction, in the majority of cases (82%), both patients and neurologists reported the same level of satisfaction with treatment. However, for 14% of patients, neurologists reported a higher level of treatment satisfaction than the patients themselves, while only a very small group of patients (4%) reported a higher satisfaction level than their neurologist.15 This suggests that some healthcare professionals may be overestimating how satisfied patients are with their current CIDP treatment.
Overall, these real-world multinational survey results reveal a strong association between patients’ satisfaction with CIDP treatment and their level of disability and fatigue. The higher the disability and fatigue level, the lower the probability of being satisfied with treatment. Some variability in satisfaction levels with CIDP treatments was also evident among different European countries. Although patients and neurologists expressed the same treatment satisfaction level in most cases, when they did not, neurologists tended to report greater satisfaction than patients.15
Conclusion
In summary, these new data presented at EAN 2025 provide important insights into CIDP and its treatment. Results from the real-world survey reveal a clear link between patients’ treatment satisfaction and their levels of disability and fatigue. Notably, only a third of patients with severe disability and less than a fifth with severe fatigue were satisfied with their current treatment. There is an unmet need for CIDP treatment options that are convenient, fast-acting, and efficacious, and provide both patients and their healthcare professionals with a higher level of treatment satisfaction. Efgartigimod PH20 SC is a new therapeutic option for CIDP recently approved in Europe that was shown to improve lower limb function in treated patients in post hoc analysis of the pivotal ADHERE trial.







