Meeting Summary
A satellite symposium titled ‘CAR T Therapy: The NEX-T Frontier in MS’ was held at the 40th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). The symposium discussed current unmet needs and future advances in the management of multiple sclerosis (MS). Underlying the symposium was the importance of early intervention with disease-modifying therapy (DMT) in people with MS. As revealed by evidence from clinical trials and real-world studies, the layering of relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA) dictates how an individual with MS may develop disability as it accumulates through different mechanistic pathways over their disease course. Considering the ‘next frontier’ in MS, the critical role of B cells in chronic CNS inflammation was described. While anti-CD20 therapies have proven efficacy for relapsing forms of MS, they have failed to prevent long-term disability in progressive MS and their chronic use is associated with increased infection risk. High doses may have greater penetrance/benefit, but a high cumulative dose of anti-CD20 therapy may be associated with increased safety concerns. CD19 or B cell maturation antigen (BCMA) CAR-T cell therapy can cross the blood–brain barrier and may allow for a deep but non-sustained B cell depletion with immune reset. Efficacy and safety results available thus far support further investigation of CAR-T cell treatments in controlled clinical trials in people with MS.
Unmet Needs in Multiple Sclerosis Management and the Potential Role of CAR-T Cell Therapy
Claire Riley, Columbia University Irving Medical Center, New York, USA, explained how studies have revealed that disability progression in MS can occur not only in association with relapse but also independent of relapse activity. This was shown by evaluating outcomes with anti-CD20 monoclonal antibody treatment in people with relapsing forms of MS and those with primary progressive MS (PPMS).1,2 Detailed analysis of outcomes from participants who received anti-CD20 therapy revealed that PIRA events were the main contributors to confirmed disability accumulation.3 Summarising these findings at the symposium, Riley stated that “the majority of progression that is mediated and diminished with anti-CD20 therapy is coming from relapse-associated worsening [RAW] while PIRA still persists close to what would be expected [without treatment]”.
Improving and Reducing Progression Independent of Relapse Activity in People with Multiple Sclerosis Without Increasing Safety Concerns
A recent study of anti-CD20 therapy in patients with relapsing MS or PPMS evaluated disability progression based on BMI quartiles.4 The greatest reductions in disability progression risk were seen in the lowest BMI quartiles. Therefore, it was speculated that a higher relative anti-CD20 exposure in lighter patients may lead to more complete B cell depletion and a more pronounced delay in disability progression than in heavier patients receiving the same dose. These results prompted a high-dose anti-CD20 (ocrelizumab) clinical study that is currently ongoing.4,5 However, Riley highlighted that “there are two sides of the coin”; i.e., although high doses of anti-CD20 therapy may have greater benefit on disability progression than lower doses, high cumulative doses of anti-CD20 therapy may be associated with increased safety concerns over time.6,7 For example, in a retrospective real-world cohort, high anti-CD20 therapy cumulative doses of anti-CD20 therapy were independently associated with increased risks of serious infections and recurring outpatient infections compared with lower doses.6 Riley confirmed that these results track with their own clinical experience; consequently, the risks of infection with long-term anti-CD20 therapy are something to actively talk about and manage, particularly in older patients.
Advantages of CAR-T Cell Therapies in Multiple Sclerosis
After establishing the unmet needs in MS regarding PIRA, Riley went on to explain the rationale behind the development of CAR-T cell therapy as a potential treatment option. In the CNS, B cells release pro-inflammatory cytokines that activate astrocytes and microglia and promote CNS inflammation.8,9 They may also form ectopic lymphoid structures within the meninges, which provide a microenvironment for the interaction of B and T cells, serve as reservoir for autoreactive B and T cell reactivation, and promote B cell maturation and proliferation against specific antigens, as well as T cell activation.8-11 “Agents that do not cross the blood–brain barrier [BBB], like our currently available monoclonal anti-CD20 agents, cannot do much to address these ectopic lymphoid structures,” Riley stated. Conversely, multiple trials using intravenously infused CD19-directed CAR-T cells have proven that CAR-T cells can cross the BBB.12 Control of autoimmune diseases might require deep B cell depletion. However, current B cell-targeting treatments do not always fully eliminate circulating B cells. CAR-T cells lead to a deep and sustained eradication of target antigen-expressing cells. Riley explained the hypothesis that CD19-directed CAR-T cell therapy may achieve deep B cell depletion in people with MS, which would allow for an ‘immune reset’ (Figure 1).13
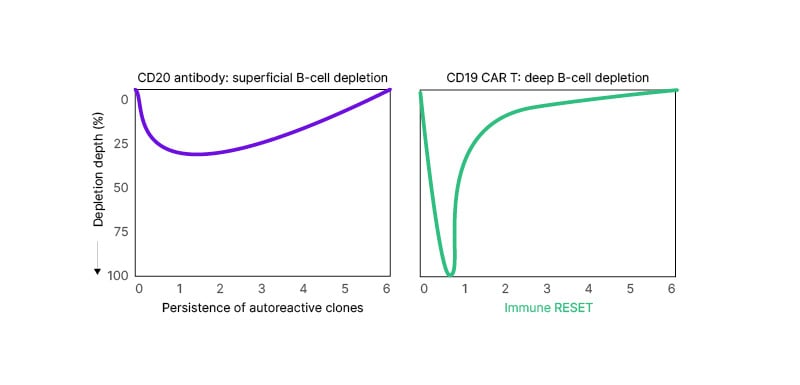
Figure 1: Hypothetical illustration of the depth and persistence of B cell depletion with a CD20 antibody versus CD19 CAR-T cell therapy.13
Treatment with a CD20 antibody results in a superficial B cell depletion, with the persistence of autoreactive clones. Treatment with CD19 CAR-T cell therapy produces a deep B cell depletion that leads to an immune reset.
Please note that the graph is a hypothetical illustration; there is no direct comparison of products.
CD: cluster of differentiation.
CAR-T Cell Therapy: How it Works and What We Have Learned in Haematologic Indications
Krish Patel, Swedish Cancer Institute, Seattle, Washington, USA, explained that a CAR-T cell recognises and binds its target antigen via the CAR and becomes activated.14,15 This initiates the cytotoxic functions of the T cell, with three main actions:
- secretion of cytokines;
- T cell proliferation; and
- secretion of proteins and activation of signalling involved in cell death.
Cytokine release activates monocytes and macrophages, which further augment the immune response.14,16 “Importantly, each CAR-T cell that is used for therapeutic purposes has a unique structure, and we can tune this structure to the purpose that we are using it for,” Patel highlighted.
CAR-T Cell Therapy is Different from Stem Cell Transplantation
Patel explained how CAR-T cell therapy differs from stem cell transplantation (SCT). Notably, SCTs typically require myeloablative conditioning with high-dose chemotherapy to destroy disease-associated cells.17 However, the conditioning regimen also destroys healthy cells, and to replace these, patients receive infusions of healthy cells via either autologous or allogeneic transplantation. SCT is usually the most successful in younger patients, as older patients are more likely to have complicated medical problems, difficulties treating graft-versus-host disease, and decreased tolerance for pre-treatment requirements.
CAR-T cell therapy combines antibody specificity with T cell cytotoxicity.14,15 It typically requires low-dose lymphodepleting chemotherapy to improve CAR-T cell expansion and proliferation.18-21 Following infusion, CAR-T cells expand and proliferate upon binding with the target antigen and so destroy harmful cells. CAR-T cell therapy can be performed in older patients without any known impact on efficacy.21
Safety Considerations with CAR-T Cell Therapy
Patel explained that CAR-T cell therapy is associated with two distinct toxicity profiles, cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which are connected to its mechanism of action.16,22 CRS is a systemic inflammatory syndrome caused by the release of high levels of cytokines after CAR-T cells bind to target cells.16,23 Symptoms of CRS include fever, myalgia, hypoxia, and hypotension, with multiple organ dysfunction possible in severe cases.16,23 ICANS is believed to occur when factors, including cytokines, cause an inflammatory state within the brain.16 Symptoms can include aphasia, delirium, tremor, lethargy, and apraxia in mild/moderate cases, and bradycardia, hypertension, seizures, and respiratory depression in severe cases.22,24 Patel explained that two key factors may influence the development and severity of CRS and ICANS. These are the costimulatory domain of CAR-T cells and disease burden. Disease burden may be a greater issue in malignancies and haematological conditions than in autoimmune and neurological diseases, where current evidence shows few cases of CRS and ICANS.
Other adverse events associated with use of CAR-T cell therapies include T cell malignancies, cytopenia, and increased risk of infection.16,20,25,26 The safety profile of CAR-T cell therapy in neurological conditions was discussed later in the session.
Cell Therapy in Neurological Diseases: Current Understandings and Potential for the Future
Final presenter, Ralf Gold, St. Josef-Hospital/Ruhr-University Bochum, Germany, shared that their institution is currently treating a ninth patient that has a neurological disease with CAR-T cell therapy.
B Cell Burden Impacts Degree of CAR-T Cell Activation and Risk of Side Effects
Following on from Patel’s observations, Gold shared key insights on how the B cell burden differs between malignancies and autoimmune/neurological conditions. B cell malignancies with a high B cell burden lead to strong activation of CAR-T cells and a relatively high risk for CRS and ICANS.27 However, in autoimmune and neuroimmunological disorders, the amount of target antigen is lower than in malignancies, leading to a lower risk for CRS and ICANS.28 In preliminary studies of CD19 CAR-T cell therapies in neurological conditions, patients experienced only Grade 1 or 2 CRS events, with no Grade ≥3 events reported.28-31
Other CAR-T cell therapies have targeted BCMA, which is expressed at later stages of B cell differentiation than CD19.32-34 This alternative B cell target may benefit patients with autoimmune diseases. According to preliminary evidence from trials of BCMA-targeted CAR-T cell therapy in neuromyelitis optica spectrum disorders (NMOSD; n=12) and myasthenia gravis (MG; n=14), all patients with NMOSD experienced low-grade CRS events and no patients experienced ICANS.35,36 None of the patients with MG experienced CRS or ICANS events.35,36
Preliminary Efficacy and Safety of CAR-T Cell Therapy in Neurological Disease
As noted above, preliminary case studies and Phase I results have been reported for CAR-T cell therapy in neurological conditions, including MG (n=14), NMOSD (n=12), stiff-person syndrome (n=1), and myelin-oligodendrocyte glycoprotein antibody-associated disease (n=1). Most patients showed no further progression of disease, with many also presenting with reduced disease activity, functional improvements, and reduced need for additional treatment. Observed cases of CRS or infections were mild; one case of Grade 1 ICANS has been reported to date.28,30,31,33-35
Gold also presented preliminary results for two individuals with progressive MS treated with CD19 CAR-T cell therapy.29 Both patients showed successful CAR-T expansion in the blood and cerebrospinal fluid (CSF) by Day 14 after treatment.
- Case 1 (secondary progressive MS): by last follow-up (Day 100), T cell numbers had declined but were still measurable.
- Case 2 (PPMS): the patient showed continued CAR-T expansion in the blood by the last follow-up (Day 28), and in the CSF until at least Day 14.
Both patients showed sustained depletion of B cells following lymphodepletion. Neither patient showed signs of disease progression as determined by EDSS score. One patient reported Grade 1 CRS; there were no reported incidences of ICANS or infections.29
Gold concluded that efficacy and safety results available thus far support further investigation of CAR-T cell treatments in controlled clinical trials in MS and other neuroimmunological conditions. However, early intervention in neurological disease (ideally in the first 6–12 months from clinical disease onset) is likely to be of importance to optimise treatment outcomes.
Panel Discussion
In answer to questions regarding the expectations for CAR-T cell therapy in people with MS, the faculty revealed their hopes for use of this innovative approach to stop or delay disease progression, including cognitive decline. Initial target populations include people with highly active disease who have not responded to initial high-efficacy DMT or those with progressive MS. Experience from, and collaboration with, colleagues in haematological and other CAR-T indications will be invaluable for optimising this therapy in the MS arena.







