Abstract
Immune checkpoint inhibitors (ICI) targeting programmed death-ligand 1 have transformed the management of advanced malignancies, yet are associated with a spectrum of immune-related pulmonary toxicities, most notably checkpoint inhibitor pneumonitis (CIP). While CIP is a well-documented immune-related adverse event, the occurrence of hydropneumothorax as a manifestation of programmed death-ligand 1 inhibitor-induced pulmonary immune-related adverse event is exceedingly rare, with very limited prior reports. The authors present a case of hydropneumothorax in a 75-year-old Hispanic male with hepatocellular carcinoma who developed respiratory symptoms 3 weeks after initiating atezolizumab and bevacizumab, following extensive prior exposure to ICIs. Hydropneumothorax occurred after initiation of atezolizumab and bevacizumab, and CIP was favoured clinically after alternative causes, including bevacizumab, prior thoracic radiation, and talc pleurodesis were evaluated. This case contributes to the growing literature by presenting one of the earliest reports of ICI-associated hydropneumothorax with histopathological characterisation, while also highlighting potential contributing factors to this complication. Clinicians should be aware of hydropneumothorax as a potential manifestation of CIP as it may require acute surgical intervention and inform future diagnostic and therapeutic strategies.
Key Points
1. The authors demonstrate one of the earliest documented cases of immune checkpoint inhibitor-associated hydropneumothorax in a 75-year-old man with hepatocellular carcinoma treated with atezolizumab and bevacizumab, expanding the spectrum of immune checkpoint inhibitor-related pulmonary toxicity.2. Pleural biopsy revealed chronic pleuritic with focal non-necrotising granulomatous inflammation, providing novel tissue-level evidence that supports an immune-mediated mechanism of injury.
3. The authors’ case highlights the need for clinicians to recognise hydropneumothorax as a potential manifestation of checkpoint inhibitor pneumonitis, which may require prompt surgical intervention and multidisciplinary management.
INTRODUCTION
Immune checkpoint inhibitors (ICI) are monoclonal antibodies capable of reversing cancer-induced immune evasion and promoting tumour death. Through blockade of critical regulatory proteins on T cells, including cytotoxic T lymphocyte antigen 4 and the programmed cell death 1/programmed cell death 1 ligand axes, ICIs have revolutionised cancer therapy and sustained remission across multiple tumour types.1,2 Toxicities stemming from ICI therapy, commonly referred to as immune-related adverse effects (irAE), are not uncommon. While most reactions are mild, some patients may experience fatal outcomes stemming from irAEs, including pneumonitis, hepatitis, colitis, neurotoxicity, and myocarditis.3 Checkpoint inhibitor pneumonitis (CIP) is defined as the new onset of dyspnoea, cough, fever, chest pain, or fatigue, alongside pulmonary exudates and evidence of interval changes in imaging not due to infection or underlying disease progression.4 Rarely, the inflammatory changes associated with CIP may be complicated by pneumothorax, with only a handful of documented case reports in the literature.5-8 Hydropneumothorax, defined by the abnormal presence of both air and serous fluid in the pleural space, is even more uncommon, with only one documented case in a patient with metastatic melanoma to the lungs.9
In this paper, the authors present the case of a patient with an extensive history of ICI therapy for treatment of hepatocellular carcinoma (HCC), with presentation of hydropneumothorax and respiratory symptoms 3 weeks after initiation of atezolizumab and bevacizumab combination therapy. To the authors’ knowledge, this is among the earliest reported cases of hydropneumothorax occurring in this clinical context with histopathological findings. Unlike prior cases, this patient had an extensive history of ICI exposure, with the complication occurring shortly after the initiation of combination atezolizumab and bevacizumab therapy. This report adds to the growing body of evidence on the pulmonary complications of ICIs by highlighting hydropneumothorax as a rare but significant and potentially life-threatening presentation of CIP.
CASE PRESENTATION
A 75-year-old Hispanic male with a history of HCC recently started on atezolizumab and bevacizumab (3 weeks prior) presented to the outpatient oncology clinic with exertional dyspnoea for 4 days. The patient was a never-smoker with no prior history of chronic lung disease including asthma, COPD, or interstitial lung disease. He was initially diagnosed with HCC after an incidental hepatic mass was detected during routine abdominal imaging. The aetiology of his HCC was attributed to non-alcoholic steatohepatitis given his risk factors, which included a past medical history of coronary artery disease, hypertension, and prediabetes. He subsequently underwent surgical resection of the mass, which was located in segment 7. Over the course of 4 years, the patient underwent multidisciplinary surveillance and management with several therapeutic interventions to treat cancer recurrence and progression. In terms of systemic therapy, he completed 6 weeks of daily lenvatinib, nine cycles of tremelimumab and durvalumab, and four cycles of nivolumab/ipilimumab combination immunotherapy followed by eight cycles of nivolumab alone. During this time, he also received stereotactic body radiation therapy (SBRT) and microwave ablation (MWA) 2 years after diagnosis, for treatment of new focal hepatic lesions, along with transhepatic arterial chemoembolisation the following year, and 18 fractions of proton therapy the subsequent year. He initiated atezolizumab and bevacizumab, and developed exertional dyspnoea approximately 7 weeks later, leading to his presentation to the oncology clinic (Figure 1).
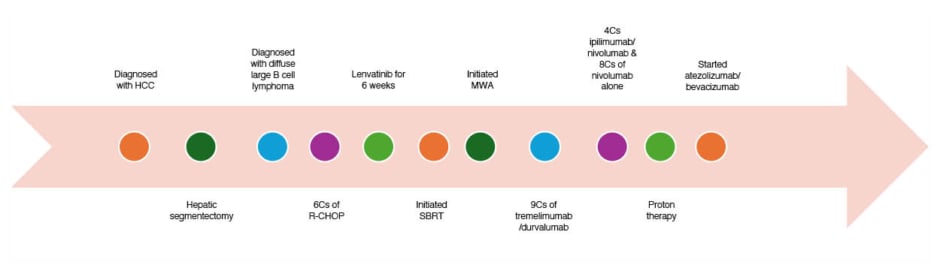
Figure 1: Clinical course of the patient.
C: cycle; HCC: hepatocellular carcinoma; MWA: microwave ablation; R-CHOP: rituximab, cyclophosphamide, doxorubicin hydrochloride (hydroxydaunomycin), vincristine sulfate, and prednisone; SBRT: stereotactic body radiation therapy; w/: with.
The symptoms began 4 days prior to presentation and were not accompanied by cough, fever, or chest pain. The patient, who permanently resides in a foreign country, stated that he noted feeling mildly short of breath while ambulating in his home. On physical examination, the patient was in no acute respiratory distress and his vital signs were within normal limits with an O2 saturation of 96% on room air. Auscultation was notable for decreased breath sounds at the right lung base, with no evidence of jugular venous distension or lower extremity oedema. A chest X-ray (CXR; Figure 2A) and CT scan of the chest (Figure 2B) showed right-sided hydropneumothorax with mediastinal shift to the left and possibility of right heart strain. Laboratory tests including white blood cell count and comprehensive metabolic panel were within normal limits and at the patient’s baseline. In the emergency department, the patient was haemodynamically stable and underwent tube thoracostomy, which was placed to -20 cmH2O suction without evidence of a persistent air leak. The chest tube remained in place for 10 days, during which interval improvements in pleural effusion and resolution of pneumothorax were observed. Pleural fluid analysis showed a lactate dehydrogenase of 305 U/L and serum lactate dehydrogenase of 162 U/L, suggesting an exudative process (Table 1). Pleural fluid and blood cultures were unremarkable for an infectious process. Cytologic evaluation was negative for malignancy with reactive mesothelial cells. Due to persistent chest output totalling 1 L and fluid re-accumulation, thoracic surgery was consulted, and the patient underwent talc pleurodesis with pleural biopsy to induce adhesion and prevent recurrent pneumothorax. Pleural biopsy specimens were obtained immediately before talc insufflation and demonstrated chronic pleuritis with focal non-necrotising granulomatous inflammation (Figure 3). No birefringent talc particles were identified, and special stains including acid-fast bacillus, Grocott methenamine silver, and periodic acid–Schiff were negative for infectious organisms. Two pleural fluid samples were also sent for cytology and not concerning for malignancy. The procedure was well tolerated, and the patient was started on systemic corticosteroids after ruling out an infectious aetiology, with subsequent symptomatic improvement. The event was classified as a Grade 3 adverse event per Common Terminology Criteria for Adverse Events (CTCAE) v5.0, as the hydropneumothorax required chest tube placement and hospitalisation.
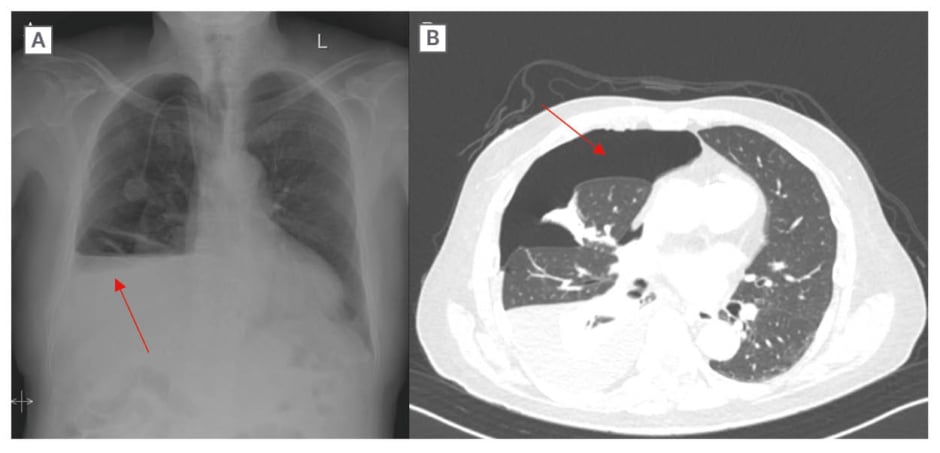
Figure 2: Radiologic findings obtained on admission.
A and B) Right-sided hydropneumothorax (red arrows); B) Near complete collapse of the right lower lobe aside and partial collapse of the right middle lobe with mediastinal shift to the left.
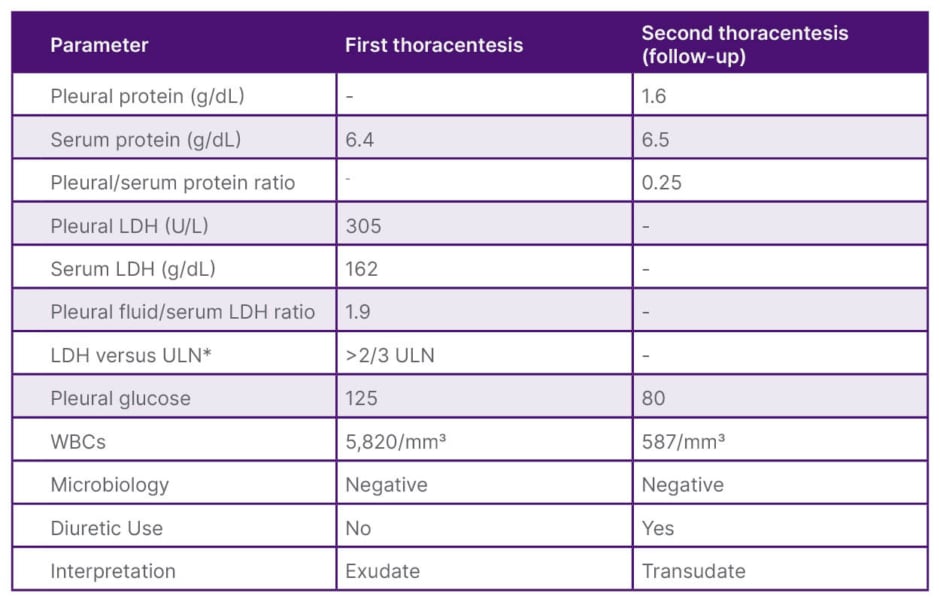
Table 1: Pleural fluid analyses from the patient’s two thoracenteses.
*ULN at the authors’ institution for serum LDH was 225 g/dL.
Light’s criteria define an exudative effusion if one or more of the following are met: (1) pleural fluid protein/serum protein ratio >0.5; (2) pleural fluid LDH/serum LDH ratio >0.6; or (3) pleural fluid LDH greater than two-thirds the ULN for serum LDH. The first thoracentesis met exudative criteria, whereas the second demonstrated transudative profile.
LDH: lactate dehydrogenase; ULN: upper limit of normal; WBC: white blood cells.
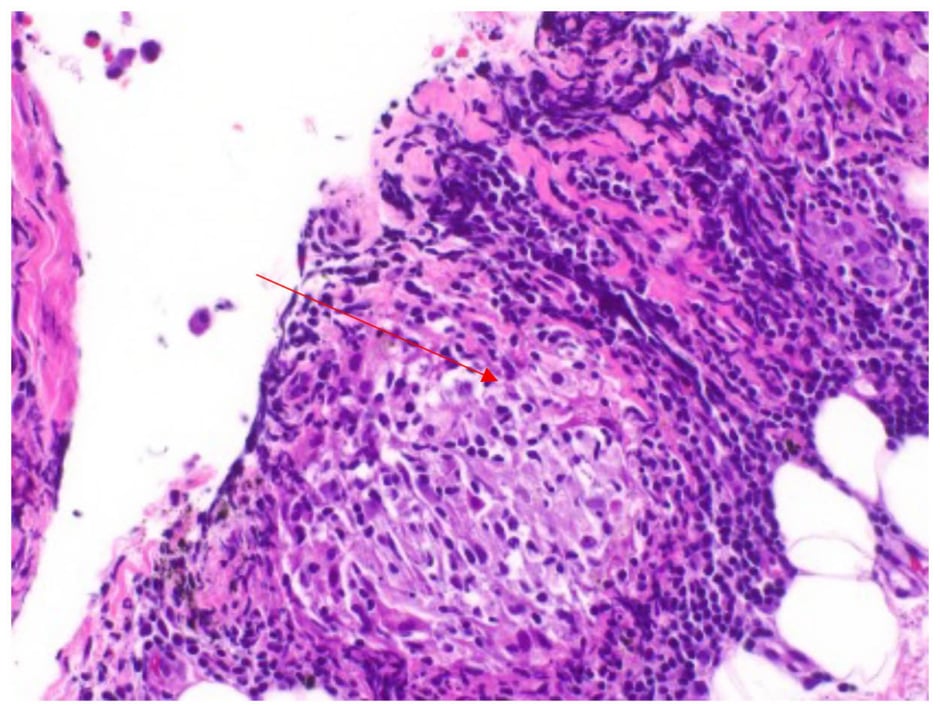
Figure 3: Pleural biopsy specimen demonstrating non-necrotising granulomatous inflammation (red arrow).
FOLLOW-UP AND OUTCOMES
Ten days after discharge, he presented to the oncology clinic endorsing improvement in shortness of breath. On physical examination, persistent decreased breath sounds in the right lung base were appreciated and a repeat CXR showed bilateral effusions increased in size compared to prior study, but no evidence of pneumothorax. During follow-up appointment 1 week later, he reported worsening shortness of breath and bilateral lower extremity swelling. A repeat CXR demonstrated stable trace bilateral pleural effusions, though there was no evidence of recurrent pneumothorax. He was started on an oral diuretic regimen for symptom control and subsequently underwent a repeat thoracentesis. Pleural fluid analysis showed a pleural protein of 1.6 g/dL, serum protein of 6.5 g/dL, and a pleural/serum protein ratio <0.5 consistent with a transudative process. The apparent shift in pleural fluid profile compared to his admission was most likely attributable to recent diuretic use, resulting in a pseudo-exudate. The patient reported improved shortness of breath during follow-up visits thereafter and has discontinued atezolizumab/bevacizumab with anticipated initiation of oral regorafenib following microwave ablation of two new focal hepatic lesions.
DISCUSSION
Hydropneumothorax is an exceedingly rare complication of ICI-related pneumonitis, with only one previously documented case in the literature.9 The authors performed a PubMed/Embase search from January 2011–May 2025 using the terms “immune checkpoint inhibitor,” “pneumothorax,” “hydropneumothorax,” and “pleural effusion,” identifying only one previously reported case of ICI-associated hydropneumothorax and several cases of pneumothorax.8-11 CIP and pulmonary toxicity associated with ICIs are well-documented in the scientific literature, with an incidence of approximately 3–5% based on systematic reviews and meta-analyses of RCTs.12,13 However, some studies suggest that the incidence may be as high as 19% in patients with lung cancer.14 While CIP typically manifests with mild pulmonary symptoms such as dyspnoea, cough, and low-grade fever, some patients progress to respiratory failure, significantly increasing their risk of mortality.3,15 Unlike typical presentations, the authors’ patient exhibited a relatively mild clinical course despite severe imaging findings. Pneumothorax remains a rare but recognised complication of CIP, with only a handful of cases reported. Given the reported CIP-associated mortality rate of 10–17%, the progression to pneumothorax or hydropneumothorax may further worsen prognosis and increase the risk of recurrence.8,16 The authors’ patient underwent surgical intervention to prevent future recurrence, placing him at increased risk for mortality both from the procedure and the underlying pathology. To the authors’ knowledge, this is among the first cases documenting histopathologic findings in a patient with ICI-related hydropneumothorax, adding novel insight into the underlying pathophysiology of this complication.
In evaluating the aetiology of the patient’s hydropneumothorax, several potential contributors were considered. CIP was favoured given the close temporal association with atezolizumab initiation, the presence of inflammatory and non-necrotising granulomas on biopsy, symptomatic improvement with steroids, and the absence of infection or tumour involvement on microbiologic and cytologic studies. However, alternative causes also merit discussion. Bevacizumab, an anti-vascular endothelial growth factor agent that is administered concurrently, may impair wound healing and predispose patients to bronchopleural fistula formation, which could increase pneumothorax risk.17 Furthermore, the patient’s history of prior thoracic interventions, including SBRT and MWA, are established cases of delayed pulmonary and pleural complications, including fibrosis and spontaneous pneumothorax months to years after exposure.18,19 Talc pleurodesis, performed at the time of biopsy, can also include granulomatous inflammation that may mimic immune-mediated pathology.20 However, this is less likely given that specimens were obtained prior to talc insufflation, and no birefringent talc particles were identified histologically. Finally, infectious aetiologies were excluded through negative bacterial, fungal, and mycobacterial cultures. Altogether, the combination of negative microbiologic work-up, lack of tumour cavitation, supportive histopathology, and temporal relationship with ICI exposure favours CIP as the most likely driver, though a multifactorial process cannot be excluded, especially in the setting of prior SBRT and MWA. The authors suspect the mechanism involved ICI-induced subpleural inflammation with subsequent air leak, compounded by impaired healing and fistula risk from concurrent bevacizumab therapy along with underlying parenchymal fragility from prior thoracic radiation.
Another novel aspect of the authors’ case was the capturing of histopathologic results from the patient’s pleural biopsy. The most common histopathologic finding in patients with CIP is organising pneumonia; however, other patterns of lung injury including acute fibrinous inflammatory changes and diffuse alveolar damage may also be seen.4,21 Nonetheless, there are currently no specific histologic findings specific for CIP. The authors’ patient’s biopsy results revealed focal non-necrotising granulomatous inflammation, which is less common in patients with ICI-related pneumonitis.21 These biopsy findings are consistent with chronic pleuritis and sarcoid-like granulomatous reactions that are well-described with ICIs, with a prior history of SBRT and MWA as plausible contributing factors. Infection and talc pleurodesis may also precipitate granulomatous reaction; however, this is less likely given the negative microbiological cultures and sampling of the pleura prior to insufflation during talc pleurodesis. Future research with a larger cohort of patients may help clarify the significance of these histopathological findings and their relationship with long-term ICI use, ultimately guiding clinicians in the identification of high-risk patients and the development of targeted therapeutic strategies to mitigate the risk of severe complications.
The precise mechanisms underlying irAEs, including pneumonitis, remain incompletely understood. It has been suggested that patients with autoimmune conditions and specific HLA mutations may be more susceptible to these toxicities and experience worse outcomes.22,23 Wang et al.24 identified inflammatory cytokines, such as serum IL-17A and IL-35, as potential biomarkers for predicting the severity of ICI-induced pneumonitis. Additionally, the association between HLA-DR4 mutations and increased risk of ICI-induced diabetes suggests a genetic predisposition to irAEs.25 Given the authors’ patient’s remote history of hypothyroidism secondary to Hashimoto’s thyroiditis, it is plausible that he had a heightened susceptibility to immune-mediated pulmonary toxicity. Current evidence suggests that irAEs may arise from increased T cell diversity, cross-reactivity between tumour and self-antigens, and imbalances between effector and regulatory T cells.26 These findings emphasise the potential benefit of pre-treatment genetic screening to identify high-risk individuals and implement personalised strategies for mitigating toxicity. In this case, the development of hydropneumothorax may have resulted from chronic pleural and parenchymal inflammation, predisposing the patient to spontaneous pneumothorax. However, due to the rarity of this presentation, further research is needed to elucidate the precise pathophysiologic mechanisms linking ICI therapy to hydropneumothorax.
One of the key considerations in this case is the relationship between the duration of ICI therapy and its prognostic implications. Most cases of CIP present early, with a median time to onset of 2.8 months from ICI initiation.27 Huang et al.28 reported that patients with early-onset CIP (within six weeks of treatment initiation) experience higher mortality rates compared to those with late-onset CIP. The authors’ patient had received multiple rounds of ICI therapy, including durvalumab, nivolumab, and atezolizumab, over 4 years before presenting with hydropneumothorax. This prolonged exposure to ICIs may have influenced his relatively stable in-hospital and perioperative course. While this case suggests that delayed onset of ICI-induced pulmonary complications may be associated with more favourable outcomes, further studies are needed to establish the clinical significance of this observation.
CONCLUSION
This case report expands the current understanding of ICI-related pulmonary toxicity by documenting hydropneumothorax as a potentially rare and severe manifestation of CIP. Histopathological characterisation of such rare complications has remained largely unexplored. The authors’ report is, to their knowledge, among the first to provide detailed histopathological evidence of chronic pleuritis with focal non-necrotising granulomatous inflammation in the setting of ICI-associated hydropneumothorax, thereby offering new insights into the tissue-level inflammatory processes underlying this complication. Clinicians should maintain a high index of suspicion for hydropneumothorax in patients with prolonged ICI exposure who develop new respiratory symptoms, as patients may require surgical intervention if there is no improvement with medical management, as in this case. Histopathological evaluation may be considered when the diagnosis is uncertain or when management decisions may be impacted. This case highlights the evolving spectrum of ICI-induced pulmonary adverse events and the importance of integrating clinical, radiographic, and histopathologic data to optimise patient care.
PATIENT PERSPECTIVE
The patient appreciated the multidisciplinary approach and felt that his concerns were addressed throughout his care. The patient noted that understanding of the potential risks and benefits of ongoing immunotherapy was important to him and his family, and he valued being involved in decisions regarding his treatment plan.







