Abstract
Cervical cancer continues to have a global impact, with an annual incidence of 600,000 cases and mortality between 300,000–350,000. Despite improved outcomes for early-stage and locally advanced disease, and decades of treatment advancements, the 5-year overall survival for recurrent or metastatic cervical cancer (RMCC) remains below 20%. This review aims to examine advances in RMCC management, current evidence, and novel research that could change future management.
Platinum-based chemotherapy combined with paclitaxel continues to have strong evidence as the backbone of first-line management. The integration of bevacizumab further prolonged survival, and the advent of immunotherapy has proved groundbreaking for RMCC prognosis. Specifically, pembrolizumab or atezolizumab may prolong survival by up to a year when added to first-line management. However, second- and third-line management of RMCC remains suboptimal. Antibody–drug conjugates, such as tisotumab vedotin, have demonstrated the most prolonged overall and progression-free survival in this setting. Future breakthroughs appear imminent in the fields of combined immunotherapy and adoptive cell therapy, with Phase I and II trial evidence suggesting promising response rates and improved overall survival, along with well-tolerated toxicity. The recent innovations in RMCC management trend towards an increasingly individualized approach. Continued investment is crucial to better understand a disease that maintains abysmal outcomes in the recurrent or metastatic setting, and remains suboptimally managed on a global scale. Fortunately, groundbreaking trials, such as KEYNOTE-826, BEATcc, and innovaTV 301, have established improved outcomes through immunotherapy and antibody–drug conjugates as standards of care in RMCC management, and continue to guide future research endeavors.
Key Points
1. Despite improvements in screening and outcomes for early-stage, localized cervical cancer, recurrent and metastatic cervical cancer continues to have suboptimal outcomes.
2. Platinum-based chemotherapy with paclitaxel remains as the longstanding backbone of first-line treatment. The integration of bevacizumab and pembrolizumab or atezolizumab into first-line treatment has significantly prolonged overall survival.
3. The KEYNOTE-058, BEATcc, and innovaTV 301 trials have provided groundbreaking research supporting improved outcomes with immunotherapy and antibody drug conjugates. Future research appears promising within combined immunotherapy and adoptive cell therapy.
INTRODUCTION
Despite improved screening and treatment in developing nations, cervical cancer continues to have a global incidence of approximately 600,000 new cases per year and a mortality of 300,000–350,000 individuals per year.1 In the United States, the estimated annual incidence and mortality were 13,800 and 4,300, respectively, in 2024.2 Fortunately, for early-stage and locally advanced disease, the 5-year overall survival (OS) is 91% and 61%, respectively.1,3 Management is nuanced and may involve extrafascial hysterectomy for Stage IA1 disease, with the addition of sentinel or pelvic lymph node dissection for Stage IA2-B1. Radical hysterectomy or external beam radiation therapy (EBRT) with brachytherapy is the approach for Stage IB2-IIA2 disease, with added systemic chemoradiation for more advanced disease.4 Locally advanced disease has been shown to benefit from EBRT and brachytherapy with radiosensitizing chemotherapy (preferably cisplatin; alternatively, carboplatin if cisplatin-intolerant).4,5
However, for up to 15% of individuals diagnosed with primary metastatic disease, or 30% of those with recurrence after treatment, the prognosis remains poor. Despite decades of advancements, treatment options have a limited impact, and 5-year OS remains below 20%.1,3,6
Recurrent or metastatic cervical cancer (RMCC) are often grouped together given their similar prognosis and management. Recurrent cervical cancer is defined as local tumor regrowth, or new nodal, or distant metastases more than 6 months after regression of the primary lesion.6 Metastatic disease is defined as spread to adjacent organs, involvement of the bladder or bowel mucosa, spread beyond the true pelvis, or distant metastasis.4 Given that recurrent and metastatic disease share similar prognoses, are frequently researched in conjunction, and share similar management guidelines, recurrent and metastatic disease, as defined above, will be addressed concomitantly in this article unless otherwise specified.
The Moore criteria were developed as a risk stratification tool to predict response of RMCC to platinum-based chemotherapy.7,8 The criteria include five risk factors independently correlated with poor response to cisplatin in RMCC: African American race, pelvic disease, performance status >0, prior radiosensitizing chemotherapy, and first recurrence within 1 year of diagnosis.7,8 The Phase III trial data from the GOG 240 trial demonstrated their validity.7 Low-risk (0–1 risk factors), mid-risk (2–3 risk factors), and high-risk (4–5 risk factors) disease were found to have significantly different OS at 21.8, 14.7, and 8.2 months, respectively (p<0.0001).7 Consequently, the Moore criteria are useful as a predictive tool, but moreover highlight the need for alternative treatment modalities, especially for patients with multiple risk factors, who are unlikely to respond to platinum-based chemotherapy in the recurrent or metastatic setting.7,8
As our understanding of HPV and cervical cancer pathophysiology continues to advance, the management of RMCC becomes increasingly disease-specific and efficacious. Notably, bevacizumab, immune checkpoint inhibitors, and antibody–drug conjugates (ADC) have all improved outcomes, and future therapeutic options are promising. As such, the objective of this review article is to examine the evidence for current recommended treatments, new advancements, and potential future approaches that could improve the abysmal prognosis of RMCC.
SURGICAL OR RADIOTHERAPY OPTIONS FOR CENTRALLY RECURRENT OR OLIGOMETASTATIC CANCER
Surgical management has a limited role in recurrent cervical cancer. Surgical resection is generally only appropriate for cases of locoregional recurrent disease or select cases of resectable metastatic recurrence. In radiation-naive patients with locoregional recurrence, surgical resection (if feasible) should be performed along with EBRT, platinum-based chemotherapy, and brachytherapy if possible.4
However, in cases with prior radiation therapy and local pelvic recurrence, pelvic exenteration is an appropriate option. It should not be considered for patients without prior pelvic radiation, unless pelvic radiation is contraindicated.4 Exenteration is a potentially curative option, with OS at 5 years ranging from 30–60%; however, its morbidity is notable: operative complications can occur in as many as 68% of cases, and re-operation is necessary for up to 45% of patients.9-13 Extensive preoperative counseling and evaluation for potential metastasis (both pre- and intra-operatively) are therefore crucial prior to exenteration. Completing intraoperative radiation therapy during exenteration is an additional option, albeit with limited data to suggest significantly improved outcomes.14 With expanding options for novel and targeted therapies for RMCC, exenteration has fallen out of favor in certain settings given the morbidity. With careful patient selection and counseling, a possible alternative could be to pursue radical hysterectomy, brachytherapy, or individualized EBRT, with or without systemic chemotherapy.4 Radical hysterectomy should be limited to patients with well-documented persistent or recurrent disease confined to the cervix, with a diameter less than 2 cm, and no evidence of metastatic disease.4
In cases of non-central recurrence, the treatment approach should be individualized based on factors such as patient goals and desires, comorbidities, and location of recurrence. Management options may include surgical resection (if feasible), with or without intraoperative radiation therapy, systemic chemotherapy, EBRT, or palliative care.4
ESTABLISHING PLATINUM- BASED DOUBLET AS THE BACKBONE FOR RMCC
Historically, the Phase III trial by Long et al.15 was pioneering in establishing superior OS with combination therapy instead of cisplatin monotherapy. Furthermore, topotecan and cisplatin were demonstrated as a viable combination regimen, as it yielded an OS of 9.4 months, compared to 6.5 months seen with historical standard of care using cisplatin monotherapy.15 Subsequently, platinum-based monotherapy is no longer recommended as first-line treatment for RMCC.4,15 The methotrexate, vinblastine, doxorubicin, and cisplatin combination was also studied, but discontinued early due to four treatment-related deaths.15
Platinum-based chemotherapy with paclitaxel has been well established as the first-line management for RMCC.4,16-20 Cisplatin has historically been the platinum-based agent of choice, with the subsequent addition of paclitaxel. Furthermore, the past 20 years of research have shown carboplatin to be a non-inferior option with a better tolerated toxicity profile.16-20
In a retrospective study of patients with Stage IVB, recurrent, or persistent cervical cancer, Moore et al.16 found no significant difference in median OS between carboplatin-paclitaxel (11 months) and cisplatin-paclitaxel (14 months). Objective response was seen in 53% and 29%, respectively, for carboplatin–paclitaxel and cisplatin–paclitaxel.16 Nevertheless, the historical first-line therapies were essentially considered palliative, given the limited OS of 7–14 months.16,21,22
The JCOG0505 Phase III trial provided further evidence of non-inferiority for carboplatin-paclitaxel compared to cisplatin–paclitaxel in RMCC, with an OS of 17.5 months and 18.3 months, respectively (hazards ratio [HR]: 0.994; 90% CI: 0.79–1.25; noninferiority p=0.032).17 Interestingly, this was not the case for patients without prior cisplatin chemotherapy, where cisplatin significantly prolonged OS compared to carboplatin (13.0 versus 23.2 months; HR: 1.571; 95% CI: 1.06–2.32).17 Consequently, cisplatin appears superior for platinum-naïve patients.
In terms of toxicity, carboplatin appears better tolerated, with decreased incidence of Grade IV neutropenia, Grade III–IV febrile neutropenia, nephrotoxicity, and nausea/vomiting, based on data from the JCOG0505 trial.16,17 A lower incidence of Grade III or above gastrointestinal toxicity with carboplatin was also observed in a 2019 cohort study.20
Ultimately, platinum-based chemotherapy with paclitaxel has been the longstanding backbone of first-line therapy for RMCC. Cisplatin specifically would be the preferable option for a platinum-naïve patients with RMCC. However, the improved toxicity profile of carboplatin-paclitaxel is an important consideration, especially in elderly or patients with multiple comorbidities, where improved tolerability may yield less frequent dose adjustments and regimen discontinuation, while maintaining a non-inferior outcome to the historical cisplatin-based regimen.
Alternative first-line chemotherapy regimens include topotecan with paclitaxel. The final outcomes from the GOG 240 Phase III trial established comparable outcomes between cisplatin–paclitaxel and topotecan–paclitaxel, with OS of 15 and 12 months, respectively (HR: 1.10; 95% CI: 0.82–1.48; p=0.52).23 The rationale was to evaluate whether topotecan–paclitaxel could improve OS in platinum-resistant RMCC; however, no significant difference in OS was found in this cohort.23 No significant differences in toxicity have been observed, but neutropenia and leukopenia appear more common with topotecan–paclitaxel, whereas nausea/vomiting, gastrointestinal, metabolic, neurosensory, and allergic adverse events were more common with cisplatin–paclitaxel.23 Topotecan–paclitaxel is thereby a reasonable alternative first-line regimen for appropriate patients, notably those with concerns for gastrointestinal, metabolic, or neurosensory adverse effects.4
APPROVAL AND INTEGRATION OF BEVACIZUMAB
An improved understanding of the human papillomavirus (HPV) pathophysiology in cervical cancer during recent decades has led to the addition of antiangiogenic therapy with bevacizumab. The E6 oncoprotein of HPV induces angiogenesis via upregulation of VEGF, and thus presents as a potential target in the treatment of RMCC.24-26 The addition of bevacizumab to first-line chemotherapy was studied in the GOG 240 Phase III trial.
The randomized controlled trial consisted of four arms: cisplatin and paclitaxel, with or without bevacizumab, and topotecan and paclitaxel, with or without bevacizumab.24,25 Final outcomes, published in 2018, demonstrated significantly prolonged OS of 16.8 months compared to 13.3 months (HR: 0.77; 95% CI: 0.62–0.95; p=0.0068), and progression-free survival (PFS) of 8.2 months compared to 6.0 months (HR: 0.68; 95% CI: 0.56–0.85; p=0.0002) with integration of bevacizumab.24,25 Notably, the addition of bevacizumab to the topotecan and paclitaxel regimen had a similarly prolonged, albeit not statistically significant, OS of 16.2 months compared to 12.0 months (HR: 0.80; 95% CI: 0.5–1.08; p=0.15). In patients without prior radiotherapy, a non-statistically significant prolonged OS with 24.5 compared to 16.8 months (HR: 0.64; 95% CI: 0.37–1.10; p=0.11) was observed.24
Furthermore, a meta-analysis of 19 studies supported a significantly increased OS with the integration of bevacizumab into cisplatin–paclitaxel or topotecan-paclitaxel. This outcome was also observed in non-platinum-based chemotherapy regimens.27 The meta-analysis found that the combination of cisplatin, paclitaxel, and bevacizumab had the highest probability of prolonging OS among first-line regimens for recurrent or metastatic disease.27 These studies, viewed in context, highlight the meaningful survival improvement that bevacizumab can add in specific populations, particularly for those without contraindications.
However, management of the unique toxicities of bevacizumab must also be considered with its use. Per GOG 240, bevacizumab has an increased incidence of Grade ≥2 hypertension (25% versus 2%; p<0.001), Grade ≥3 thromboembolic events (8% versus 1%; p=0.001), and genitourinary or gastrointestinal fistulas (15% versus 1%), and Grade 3 fistulas requiring intervention (6% versus 0%; p=0.002) when compared to placebo. Clinically relevant fistulas only occurred in previously irradiated patients. There was no difference in the rate of fatal adverse events with or without bevacizumab addition.24,25
Ultimately, the integration of bevacizumab into first-line chemotherapy for RMCC should be recommended for all appropriate patients, as it may prolong survival by multiple months. Previously irradiated patients should be counseled on the increased risk of fistula formation. Furthermore, bevacizumab may not be appropriate for patients with history of gastrointestinal perforation, active bowel obstruction, poorly controlled hypertension, or recent thromboembolic events, cerebrovascular accidents, or cardiovascular disease.
Recent research has also introduced other antiangiogenic agents into RMCC management. Cediranib is an oral tyrosine kinase inhibitor of VEGFR1, 2, and 3. A randomized, double-blind Phase II trial comparing carboplatin and paclitaxel with cediranib versus placebo in RMCC found that PFS in the cediranib group was longer (median: 8.1 months) than in the placebo group (6.7 months; p=0.032), with increased toxicities, including diarrhea, neutropenia, and hypertension.28 However, this trial was terminated early due to the loss of drug supply, and cediranib is not FDA-approved for RMCC.
The VEGFR2 tyrosine kinase inhibitor, apatinib, has shown in vitro response and an OS of 12.3 months with a 15% response rate in early Phase II RMCC trials.29,30 Although strong evidence is lacking for apatinib, it follows the trend of increased integration of antiangiogenic agents in cervical cancer. This highlights how an improved understanding of HPV pathophysiology and angiogenesis has been translated into improved outcomes.24-26
APPROVAL OF IMMUNOTHERAPY FOR RMCC
There has been a recent paradigm shift in the management of RMCC, with increased availability of testing for specific tumor biomarkers, allowing for an individualized treatment approach.
The monoclonal antibody targeting PD-1, pembrolizumab, has now been designated by the National Comprehensive Cancer Network (NCCN) as first-line treatment for PD-L1 positive (combined positive score [CPS] ≥1) RMCC, in combination with first-line chemotherapy and bevacizumab.4 This update is based on the KEYNOTE-826 Phase III trial, which showed significantly improved PFS (10.4 versus 8.2 months; HR: 0.65; p < 0.001) and OS at 24 months (50.4% versus 40.4%; HR: 0.67; p<0.001) when pembrolizumab was added to current first-line chemotherapy.31
Long term follow-up from the KEYNOTE- 826 trial further supported the utility of pembrolizumab integration. With a median follow-up of 39.1 months, the median OS for pembrolizumab versus placebo, respectively, was 28.6 and 16.5 months (HR: 0.62; 95% CI: 0.49–0.74) in the PD-L1 CPS > 1 group, 26.4 and 16.8 months (HR: 0.63; 95% CI: 0.52–0.77) in the all-comers group, and 29.6 and 17.4 months (HR: 0.58; 95% CI: 0.44–0.78) in the PD-L1 CPS > 10 group.32 In other words, pembrolizumab prolonged OS by approximately one year for patients with PD-L1 expression, and 10 months regardless of PD-L1 status.
The addition of pembrolizumab to chemotherapy is recommended as first-line management for recurrent or metastatic tumors with PD-L1 expression with CPS ≥1.4 Pembrolizumab should be considered for all patients, as PD-L1 expression (CPS ≥1) is present in most RMCC tumors. KEYNOTE-826 found PD-L1 with CPS ≥1, in approximately 90% of cases, with the lower end of prevalence around 63.8–77.8% in other studies.33,34 Although pembrolizumab has not shown a survival benefit for CPS <1, it should be considered if PD-L1 status is unknown or unavailable, as PD-L1 overexpression is prevalent in cervical carcinomas, and KEYNOTE-826 demonstrated a significantly prolonged OS even in the all-comer group.31,32
Similarly, the checkpoint inhibitor targeting PD-L1, atezolizumab, was recently approved as an immunotherapy addition to first-line RMCC management. Based on the findings from the BEATcc trial, the addition of atezolizumab to cisplatin or carboplatin-paclitaxel-bevacizumab prolonged OS (32.1 versus 22.8; HR: 0.68; 95% CI: 0.52–0.88; p<0.0046) and PFS (13.7 versus 10.4 months; HR: 0.62; 95% CI: 0.49–0.78; p<0.0001).35
Because of the recent breakthroughs with immunotherapy, NCCN guidelines began to issue Category 1 recommendations for first-line treatment of RMCC with atezolizumab with paclitaxel, cisplatin or carboplatin, and bevacizumab. For patients with PD-L1 expression (CPS ≥1), pembrolizumab with paclitaxel, platinum-based agent, and bevacizumab is the preferred treatment, supporting by Category 1 evidence.4,31,32,35
The promising outcomes of immunotherapy have proposed the potential of combination immunotherapy. Early in vitro research demonstrated significantly impeded cervical cancer cell proliferation with combination of lenvatinib (a tyrosine kinase inhibitor) and a PD-1/PD-L1 inhibitor, relative to the individual agents in isolation.36 The combination of CTLA-4 and PD-1 checkpoint inhibition, by zalifrelimab and balstilimab respectively, was examined in a large Phase II trial by O’Malley et. al.37 for patients with RMCC after prior platinum-based therapy. An overall response rate of 25.6% (95% CI: 18.8–33.9) was observed, and the median duration of response was not reached in 125 patients after a median follow-up period of 25 months.37 Dysregulated thyroid function was the most common adverse effect.37 Phase III trials will be helpful to compare these outcomes to single-agent immunotherapy and other second-line therapies.
SECOND LINE AND BEYOND
There are only two preferred second-line treatment regimens for RMCC with Category 1 evidence: tisotumab vedotin for all patients, or pembrolizumab monotherapy in the setting of tumors with PD-L1 positive status (CPS ≥1), high tumor mutational burden (TMB-H; ≥10 mutations per megabase), microsatellite instability high (MSI-H) or deficient in DNA mismatch repair (dMMR).4,38-40 However, there are numerous other second-line agents that could have therapeutic benefit, albeit with limited evidence and consistency.4
Single agent pembrolizumab in the second line should be limited to tumors with MSI-H/dMMR, TMB-H, or PD-L1 positive status. The evidence is based on the KEYNOTE-028 (Phase Ib) and KEYNOTE-158 (Phase II) trials.41,42 This data is limited as KEYNOTE-028 demonstrated an overall response of 17% in PD-L1 positive tumors but had a small cohort size and the approximately 30.8% overall response rate with MSI-H/dMMR tumors in KEYNOTE-158 included only nine individuals with cervical cancer.40-42 Thereby, pembrolizumab monotherapy remains a viable second line option for appropriate patients but data on outcomes is highly limited.
Cemiplimab, an antagonist antibody of PD-1, has recently been supported as a second-line treatment for RMCC.4,33,43 In the EMPOWER-Cervical 1 Phase III trial, cemiplimab demonstrated a significantly longer OS (11.7 versus 8.5 months) compared to physicians’ choice single-agent chemotherapy in patients with disease progression after first-line platinum-based chemotherapy (HR: 0.67; 95% CI: 0.56–0.80; p<0.00001). This benefit was observed in both squamous and adenocarcinoma subtypes. Grade ≥III toxicity was also less frequent with cemiplimab (45.0% versus 53.4%).33,43 Notably, the OS benefit was observed regardless of tumor expression of PD-L1, making cemiplimab a strong option for all patients with progression after platinum-based therapy.43
In addition, nivolumab has emerged as an alternative PD-1 targeting agent with promising outcomes. Data from the Phase I/II CheckMate 358 trial have shown some early promise for nivolumab in the treatment of HPV-mediated tumors. Among the 19 patients with cervical cancer, who had undergone prior first-line chemotherapy for metastatic disease, there was a 26.3% objective response rate (ORR; 95% CI: 9.1–51.2), and median OS of 21.9 months.44 Although promising, further research is needed, and nivolumab remains a recommendation in select cases of PD-L1 positive tumors as second-line therapy.
Single agent chemotherapy with agents such as bevacizumab, paclitaxel, topotecan, gemcitabine, docetaxel, pemetrexed, and irinotecan remains a potential therapy in recurrent, persistent, or metastatic RMCC. However, they are not preferred regimens due to relatively poor supporting evidence.4 For instance, early trials of paclitaxel monotherapy demonstrated a 17% response rate, with neutropenia as a common dose-limiting toxicity.45 Nevertheless, these single agent regimens have repeatedly been shown to have relatively shorter OS and PFS in comparison to aforementioned combination regimens and newer immunotherapies and ADC. As such, single agent regimens should be reserved for patients who have exhausted other appropriate management options.
THE NEW AGE: ANTIBODY-DRUG CONJUGATES IN RECURRENT OR METASTATIC CERVICAL CANCER
As previously mentioned, tisotumab vedotin (Tivdak®; Pfizer, New York, USA) is currently the only targeted therapy regimen with Class I evidence for second-line management of RMCC.4 Tivdak is the first-in-human ADC for RMCC, where the monoclonal antibody binds to the tissue factor on tumor cells, which is typically overexpressed in RMCC. This allows entry of the covalently bonded complex, where Tivdak undergoes proteolytic cleavage to release monomethyl auristatin E, which induces microtubule disruption and inhibits cell division.38,46,47 Initial Phase I and II trial data from innovaTV 201 and 204, showed a 24% ORR (95% CI: 16–33) with 7% complete and 17% partial responses, over a 10-month median follow-up.38,47 Grade ≥III toxicity occurred in 28% of patients (neutropenia 3%, fatigue 2%, ulcerative keratitis 2%, and peripheral neuropathy 2%).38 The innovaTV 206 trial further supported the safety profile and objective response.46
The subsequent Phase III innovaTV 301 trial demonstrated promising evidence when compared to physician’s choice chemotherapy in the second- and third-line setting, with significantly prolonged OS (11.5 versus 9.5 months; HR: 0.70; p<0.004), PFS (4.2 versus 2.9 months; HR: 0.67; p<0.001), and improved objective response (17.8% versus 5.2%; odds ratio: 4.0; p<0.001).39 Thereby, tisotumab vedotin, if tolerated, should be considered as the preferred agent for patients with RMCC in the second- and third-line setting, given a 30% reduction in mortality, and prolonged OS of 2 months.39 Tivdak also appears to be better tolerated than other agents in this setting, with fewer Grade ≥III toxicities (52.0% versus 62.3%), and only a 14.8% discontinuation rate based on adverse events.4,39 Notably, ocular toxicity appears to be a common adverse effect of Tivdak, affecting 31.2% of patients, compared to 0.4% in controls.39
Furthermore, data from the innovaTV 205 demonstrated promising response rates when Tivdak was combined with carboplatin (54.5%), pembrolizumab (40.6%), and bevacizumab (35.3); thus, highlighting a role for combination therapy in future guidelines given additional Phase III data.48 Furthermore, more diverse trials may be needed, given the significant underrepresentation of Black patients in innovaTV 301, and this population has worse RMCC outcomes.39
HER2 was demonstrated as a target for the ADC trastuzumab deruxtecan, in the management of breast, gastric, and non-small cell lung cancers. It has now shown efficacy in the treatment of cervical carcinoma, among other solid tumors, based on the DESTINY-PanTumor02 Phase II trial.49 This trial examined tumors with HER2 immunohistochemistry (IHC) of 3+/2+, or metastatic disease after one or more failed prior systemic treatments, and found an overall response rate of 37.5% for cervical carcinomas (95% CI: 22.7–54.2) across a median 12.75 month follow-up period. In addition, OS and PFS were prolonged, most notably for the IHC 3+ cohort.49 HER2 is expressed in 3–6% of cervical carcinomas and associated with poor outcomes.50 It appears that trastuzumab deruxtecan could exhibit benefit in the second-line treatment setting, with the most significant impact in tumors with HER2 expression levels of 3+ as determined by IHC, and confirmed by fluorescence in situ hybridization analysis if indicated. However, as there are no Phase III trials, there is limited evidence for its generalized clinical applicability at this time, and it remains limited as a potential option in select clinical situations and research trials.
Similarly, the pan-HER tyrosine kinase inhibitor, neratinib, was evaluated for treatment of RMCC, following progression/recurrence after prior platinum-based chemotherapy in the SUMMIT basket Phase II trial. In a small cohort of 22 patients, objective response was observed in 22.2% of cervical adenocarcinomas, whereas, no objective response was observed in patients with squamous cell carcinomas.50 Similarly, PFS was significantly prolonged at 5.5 months for adenocarcinomas, with no significant PFS impact with squamous cell tumors.50 Although, neratinib appears to have potential efficacy against recurrent/metastatic adenocarcinomas, its application may be limited to patients with adenocarcinoma histology and who are not candidates for alternative options such as pembrolizumab or Tivdak, given the stronger evidence for these agents. Furthermore, the prolonged OS and PFS observed with cemiplimab in the second line setting for RMCC is better evidenced currently, regardless of PD-L1 status.33,43 The lack of evidence from a Phase III trial further prohibits evidence-based utility on a large scale, and the clinical benefit of neratinib remains hypothetical at this time.
In recent years, ADC are providing further options for targeted therapy based on tumor histology, and promise for better outcomes for patients with RMCC after failed prior treatments. Agents like sacituzumab govitecan, which targets Trop-2 and delivers irinotecan into tumor cells, has shown promising potential in vitro.51 However, clinical trials are needed, which highlights the importance of further research on RMCC.
Among ADC and immunotherapy agents, pembrolizumab, atezolizumab, cemiplimab, and tisotumab vedotin have arguably had the greatest impact on clinical practice and RMCC prognosis. Current NCCN guidelines reflect the significantly prolonged OS and PFS offered by these agents, as demonstrated in Table 1. Ultimately, these groundbreaking trials highlight the trend towards targeted therapy, yielding notably improved prognoses in relation to historical platinum-based chemotherapy exclusively, with the potential to improve RMCC outcomes. It appears that future investments, in identifying tumor-specific targets, may continue to advance and prolong the care and prognosis for patients with RMCC.
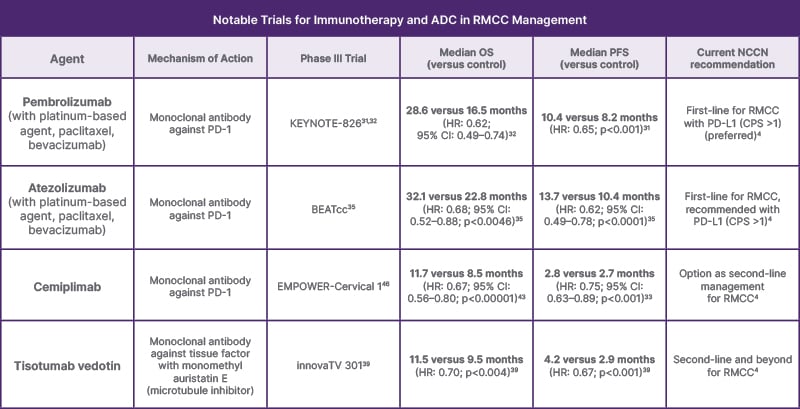
Table 1: Summary of the findings in terms of overall survival, and progression-free survival, from practice-changing Phase III trials in recurrent or metastatic cervical cancer management.
The mechanism of action for each agent is briefly detailed. The current NCCN guidelines are reflected in column number six (from the left) to demonstrate the effect of the trial on current practice.
ADC: antibody–drug conjugates; CPS: combined positive score; HR: hazard ratio; NCCN: National Comprehensive Cancer Network; OS: overall survival; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; PFS: progression-free survival; RMCC: recurrent or metastatic cervical cancer.
THE FUTURE
Innovations and a better understanding of the molecular biology of cervical cancer may provide insight into new therapies. For instance, neurotrophic tyrosine receptor kinase (NTRK) fusions may be rare in cervical cancer (0.36–1.88% of tumors); however, these tumors tend to respond poorly to chemoradiation. NTRK fusions result in constitutively activated tyrosine kinase pathways, which have a synergistic oncogenic effect with HPV-associated E6 and E7.52 Larotrectinib, entrectinib, and repotrectinib have all shown efficacy against NTRK fusion tumors, but the evidence for their implications in cervical cancer is scant to nonexistent, highlighting the need for further research.53
A potential paradigm shift of RMCC management may be on the horizon with the introduction of adoptive cell therapy (ACT). The concept involves a T cell-mediated anticancer response based on recognition of tumor-specific antigens combined with human leukocyte antigen and coactivation by B7 and CD28. Tumor cells may develop resistance by facilitating an immunosuppressive milieu to evade detection. Thus, the concept of ACT is to extract autologous T cells with specific adaptive targets to the tumor antigens, promote their proliferation ex vivo, and reintroduce them with growth factors such as IL-2 to promote a large-scale anti-neoplastic immune response.54 Genetic modification in vitro with T cell receptor modified T cells or chimeric antigen receptor T cells are also components that may be integrated to individualize ACT.54 Early studies demonstrated near 60% ORR in malignant melanomas. Recent outcomes with ACT in cervical cancer have demonstrated an ORR of 33.3–44.0%, and disease control rate of 65.4–85%.54,55 The integration of lymphodepletion in conjunction with ACT also appears to nearly double the ORR and disease control rate, theoretically by reducing the regulatory/inhibitory immune response, and warrants further research. Although, ACT is generally well tolerated and associated with few incidences of dose-limiting toxicities (6.1%), Grade 3–4 toxicities were observed in 46.0% of ACT regimens for gynecologic malignancies.54 Nevertheless, ACT could be revolutionary in improving the relatively poor outcomes of RMCC. Notably, the combination of immunotherapy with ACT may hold immense potential, given many of the mechanisms of tumor resistance to ACT involve upregulation of immunologic inhibition with CTLA-4 and PD-L1. Early data on pembrolizumab combination with ACT demonstrated a remarkable 50.0% ORR in a small cohort of 10 patients.56 While ACT appears promising as a future breakthrough to improve RMCC outcomes while reducing toxicity, its technical complexity, along with the subsequent expertise and resources requirements, may prove obstacles to larger-scale implementation and availability. In addition, limited availability may further perpetuate existing socioeconomic inequalities in cancer care and outcomes.
Bacteriophage-based therapy is another potential approach that has shown promise but faces challenges related to delivery, such as diffusion and clearance by the reticuloendothelial system. Bacteriophages are viruses that infect and proliferate via bacteria. However, they have potential to infect eukaryotic cells, such as tumor cells. Given the growth of genetic modification, such as CRISPR-Cas9, they could theoretically be modified to selectively infect and exert anti-tumor effects with exceedingly high specificity.57 The two main approaches are: to use phage therapy to introduce a specific antigen to tumor cells, which then be presented via major histocompatibility complex as a target for immune response, potentially concomitant with ACT; alternatively, to use phage therapy as a targeted delivery system for either therapeutic agents or gene therapy.57 Interestingly, a murine study using a cytomegalovirus-based (CMV-based) recombinant bacteriophage delivery of the gene for HPV16 E7 antigen, demonstrated significant inhibition of tumor growth and demonstrated therapeutic potential against HPV-associated tumors.58 Unfortunately, further research in this realm has been scarce, even though phage therapy could offer a hypothetically improved toxicity profile compared to current management options.57 Given the lack of clinical research, it remains important to emphasize the exclusively hypothetical concept of bacteriophage therapy, with no role in current clinical management.
CONCLUSION
Despite decades of advancements in treatment of RMCC, platinum-based chemotherapy has maintained its role as the backbone of first line management. The historically poor outcomes have, however, been significantly improved with the addition of anti-angiogenic agents and immunotherapy targeting PD-1 and PD-L1. The anti-angiogenic agent bevacizumab has been shown to significantly prolong OS by multiple months. Moreover, PD-1 and PD-L1 inhibition, by pembrolizumab and atezolizumab, may prolong survival by up to a year in patients with PD-L1 expressing tumors, which appears to be the case for most patients. Combination immunotherapy appears promising in vitro and in early Phase I and II trials but requires more evidence for widespread implementation. The combined result of the past few decades of advancements has significantly changed the outlook on first-line management of RMCC.
Unfortunately, second-line management continues to be limited. Pembrolizumab monotherapy has limited benefit for selecting patients with PD-L1 expression, TMB-H, or dMMR/MSI-H tumors. The most promising approach to second-line management appears to be ADC, which allow targeted delivery of the active agent by an antibody conjugate targeting tumor-specific receptor. Most notably, tisotumab vedotin has been shown to prolong OS by 2 months in the second- or third-line treatment setting, with an improved toxicity profile compared to other agents. Among other ADC, trastuzumab deruxtecan stands out for a 37.5% overall response, but it lacks Phase III clinical data. Second-line immunotherapy agents include the anti-PD-1 and anti-PD-L1 agents cemiplimab and nivolumab, which demonstrate modest potential (limited to adenocarcinoma with nivolumab). The remaining second- and third-line options consist of a wide range of single agent regimens, most of which are supported by scarce research or limited data for improved outcomes.
The hope remains that future research will provide additional groundbreaking advancements, similar to the advent of immunotherapy. A notable mention includes the introduction of ACT, with associated genetic modification (T cell receptor and chimeric antigen receptor T cell), which could produce a targeted adaptive immune response specifically against the unique tumor of the patient, by using autologous T cells modified in vitro. Although this modality remains experimental, early findings suggest impressive ORRs and disease control rates, with a well-tolerated toxicity profile. The clinical application of ACT could, however, face challenges related to cost-effectiveness and availability, and may therefore demand further research in these domains prior to realistic implementation into clinical care. Hypothetical limitations in access may further perpetuate racial and socioeconomic inequalities in RMCC care, and should also be emphasized in future research. In parallel, the hypothetical implementation of bacteriophage-based therapy is also intriguing, as it could be utilized as a directed vehicle to introduce treatment agents, treatment targets for major histocompatibility complex expression, or even genetic modifications. Murine studies have shown potential efficacy against HPV-associated tumors, but phage therapy remains far from clinical implementation and highly speculative until further research is conducted.