Editorial Note: This article, originally published on 05.09.2025, was temporarily removed for further review at Astellas’ request on 25.09.2025. It was reviewed and republished with updated PI/AER information on 25.09.2025.
Abstract
Aim: To provide practical recommendations to support the use of enfortumab vedotin combined with pembrolizumab (EV+P) for the first-line treatment of adult patients with unresectable or metastatic urothelial carcinoma (mUC) eligible for platinum-based chemotherapy who present with comorbid conditions.
Method: An international advisory panel of experts was convened to provide input into the development of these recommendations. The panel reviewed representative clinical scenarios involving patients with mUC and discussed available evidence, as well as their clinical experience, to determine key practical considerations before and during EV+P administration.
Results: Key recommendations for patients with peripheral neuropathy, skin toxicities, diabetes/hyperglycaemia, impaired renal function, frailty, obesity, and ocular disorders were presented. EV+P is the standard-of-care first-line treatment for patients with unresectable or mUC who are eligible for platinum-based chemotherapy, and patients who meet its approved indication (per the Summary of Product Characteristics) should be able to have access to it without unnecessary clinical restrictions. The expert panel considered that clinicians must familiarise themselves with its safety considerations and adverse event management strategies, especially in potentially challenging scenarios such as its use in patients with baseline comorbidities. Best practice was regarded as initiating EV+P at the recommended starting dose, with dose modifications as required.
Conclusion: Clinical judgment and shared decision-making are key to help optimise EV+P treatment, especially in patients with complex clinical profiles.
INTRODUCTION
mUC is an aggressive disease with a poor prognosis;1 the 5-year relative survival rate for patients with metastatic disease is 9.1%.2 Patients with mUC are often elderly and/or have comorbidities that negatively impact morbidity and mortality,3 such as hypertension, cardiovascular disease, and diabetes.4
Today, the main options included in the European Society for Medical Oncology (ESMO) guidelines for first-line (1L) treatment of mUC include EV+P, platinum-based chemotherapy (PBCT) followed by maintenance avelumab (if progression-free), and nivolumab with gemcitabine and cisplatin.5 Furthermore, due to high attrition rates between treatment lines, a limited proportion of patients receive second- or third-line therapy in real-world practice.6 Consequently, 1L treatment choice is critical in mUC, and the regimen with the highest potential for clinical benefit should be delivered upfront.
Today, EV+P is the preferred 1L treatment for patients with unresectable or mUC who are eligible for PBCT.5,7 This is due to the results of the primary analysis from the registrational Phase III trial, EV-302.8 Moreover, long-term data were consistent with the primary analysis results, and the median overall survival more than doubled with EV+P versus PBCT at a median follow-up of approximately 2.5 years (hazard ratio: 0.51; 33.8 months versus 15.9 months, respectively; p<0.00001). Clinical benefits observed in the intention-to-treat population were consistently observed across all prespecified subgroups (subgroup analyses in EV-302 were exploratory in nature; the study was not powered to detect differences between treatments based on prespecified subgroups), including in patients with upper tract primary disease, patients with liver metastases, and those ineligible for cisplatin.9 The incidence of Grade ≥3 adverse events (AE) was lower in the EV+P group versus the PBCT group, and the safety profiles were distinct. In the EV+P group, treatment-related AEs of special interest that have previously been associated with EV included peripheral neuropathy (PN), skin toxicities, diabetes, and ocular disorders, while the most common AE associated with PBCT was cytopenia.8
Given the adoption of EV+P as standard of care for 1L treatment of mUC, clinicians must familiarise themselves with its safety considerations and AE management strategies, particularly in the context of patients with comorbidities or frailty. Although the inclusion and exclusion criteria of the EV-302 trial were appropriate for assessing efficacy and safety in a controlled setting, they should not be used alone to guide clinical decision-making. In real-world practice, treatment decisions should be informed by the approved Summary of Product Characteristics (SmPC) indication. In addition, while clinical criteria such as the Galsky criteria are relevant to determine cisplatin eligibility,10 no similar, evidence-based criteria are established for EV+P. Patients who meet the approved indication of EV+P should be able to have access to it without unnecessary clinical restrictions.5 This is important to keep in mind, as patients enrolled in clinical trials are often younger and without comorbidities versus patients in real-world clinical settings.11 These factors can affect treatment tolerability and clinical outcomes. This highlights the importance of careful clinical assessment and appropriate treatment management at initiation and throughout the treatment course, particularly in patients with medically complex profiles, to support sustained clinical benefit. Clinicians without prior experience of treating patients with EV (either as a monotherapy or in combination) may require support (in addition to the guidance detailed in the respective SmPC)12 when making treatment decisions and managing AEs.
Accordingly, clinicians may benefit from practical guidance to support the use of EV+P in routine clinical practice, particularly regarding the management of patients presenting with pre-existing comorbidities and treatment-related AEs. This includes guidance on pre-treatment considerations, monitoring during treatment, and dose modifications for AEs of special interest (AESI), with the overall aim being to minimise the impact of treatment-related AEs and ensure optimal integration of EV+P into clinical practice.
METHODOLOGY
The objective of this article is to provide practical recommendations to support the use of EV+P in patients with clinically complex profiles. Two priorities were chosen: 1) pre-treatment considerations for patients with clinically relevant, pre-existing comorbidities; and 2) strategies to manage selected AESIs associated with EV+P.
An international advisory panel of eight medical oncologists from Europe and the USA was convened on the 11th of March 2025 to provide input into the development of these recommendations. Experts selected were either involved in the EV-30113 or EV-3028 trials, or had substantial clinical experience with EV+P.
The panel reviewed seven representative clinical scenarios involving patients with mUC. For each clinical scenario, they discussed available evidence (drawing on the SmPC and relevant published data) and their clinical experience to assist with practical considerations prior to and during EV+P administration.
The resulting key recommendations were developed and refined during a 4-hour roundtable discussion in a qualitative manner. The panel agreed with the final recommendations, which are presented in the following sections.
As EV is approved for 1L treatment in combination with pembrolizumab, some AEs, such as skin toxicities, are associated with both pembrolizumab and EV;12,14 AEs related to pembrolizumab are not discussed in this article. For details on AEs relating to pembrolizumab and their suggested management, please refer to the pembrolizumab SmPC.14
Patients with Unresectable or Metastatic Urothelial Carcinoma and Baseline Peripheral Neuropathy, or Who May Be at Risk of Developing Peripheral Neuropathy
PN is a known AESI associated with EV, primarily manifesting as peripheral sensory neuropathy.12,15 Risk factors for PN include comorbidities such as diabetes, older age, and spinal involvement of mUC, or non-malignant spinal disease.15 PN is also an established AE associated with PBCT.16
In the pooled safety population of 564 patients who received EV+P in EV-302 and EV-103, PN was the second most common AE, occurring in 67% of patients (Grade 3: 7%).15 The majority of events reported were categorised as peripheral sensory neuropathy (any grade: 53.4%), and peripheral sensory neuropathy was the most common adverse reaction leading to treatment discontinuation (12.2% of patients).12 In this pooled analysis, the median time to onset of Grade ≥2 PN was 6 months (range: 0.3–25.0). Of patients who experienced PN, with data regarding whether resolution was achieved (n=373), 13% experienced complete resolution, with 87% experiencing residual PN. Of the patients with residual PN at last follow-up, 45% had Grade ≥2 PN. Among patients in EV-103 who experienced PN, 70% had an improvement or resolution of symptoms at 4 years of follow-up.15,17
Treatment with EV+P should be initiated per the SmPC guidance. Patients should be monitored for new or worsening symptoms of PN. For patients who experience Grade 2 PN, EV should be withheld until Grade ≤1. For a first occurrence, treatment should resume at the same dose level, but for a recurrence, withhold until Grade ≤1, then reduce the dose by one level and resume treatment. For a summary of EV dose levels, see Tables 1 and 2. EV should be permanently discontinued for Grade ≥3 PN.12
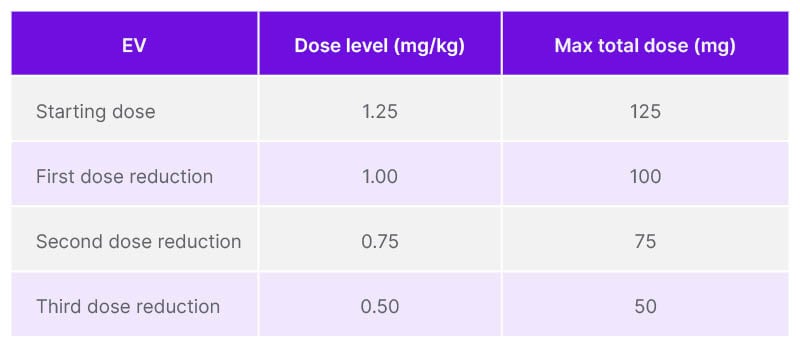
Table 1: Recommended dose reductions of enfortumab vedotin for adverse reactions, per the enfortumab vedotin Summary of Product Characteristics.
EV: enfortumab vedotin.
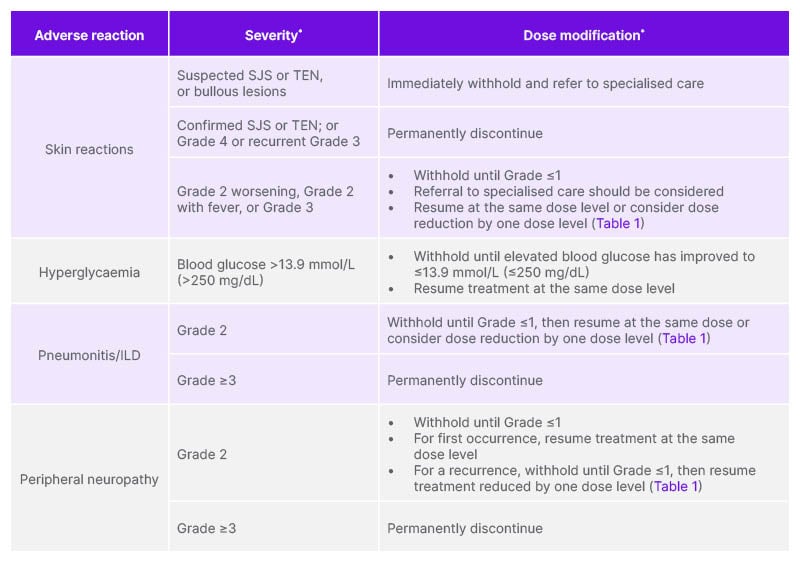
Table 2: Recommended dose modifications of enfortumab vedotin for patients with unresectable/metastatic urothelial cancer, per the enfortumab vedotin Summary of Product Characteristics.
*Toxicity was graded per NCI-CTCAE v5.0: Grade 1: mild; Grade 2: moderate; Grade 3: severe; Grade 4: life-threatening.
ILD: interstitial lung disease; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; SJS: Stevens–Johnson syndrome; TEN: toxic epidermal necrolysis.
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and baseline peripheral neuropathy, or who may be at risk of developing peripheral neuropathy
The panel advised to assess PN at baseline, with a focus on how PN impacts patients’ daily activities (through assessment of fine motor skills, gait, and balance). Assessment should include a complete medical history and assessment of any risk factors that may impact PN, such as older age, spinal involvement of mUC, diabetes, etc. The potential impact on quality of life and daily activities should be discussed with patients and considered on an individual basis, alongside the potential clinical benefits of EV+P. The panel stated that patients may be reluctant to report PN for fear of subsequent treatment interruption or discontinuation. Patients should be educated on the use of dose modifications and management strategies, as early recognition of PN and appropriate dose modification can help increase the likelihood of PN resolution and, therefore, remaining on treatment.15 Notably, a post hoc analysis of EV monotherapy studies18 and an exploratory analysis of EV-30219 indicated that recommended dose modifications are effective for managing EV-related AEs and may allow patients to remain on treatment.18,19
Treatment with EV+P should be initiated per the SmPC guidance. Patients should be informed about the signs and symptoms of PN to closely monitor for new or worsening symptoms, which should be reported to their healthcare professional (HCP) immediately. HCPs should also monitor for symptoms of PN at each visit and be aware that the onset of PN may become more likely over time (based on the median 6-month time to onset of Grade ≥2 PN in the pooled safety population).15
In the event of PN, the panel considered that HCPs should be guided by SmPC recommendations for management of these patients (Table 3). When PN is unlikely to be related to treatment with EV, consultation with a neurologist may be appropriate. In cases where PN is likely treatment-related, following SmPC guidance regarding PN resolution and EV dose modifications is recommended.12
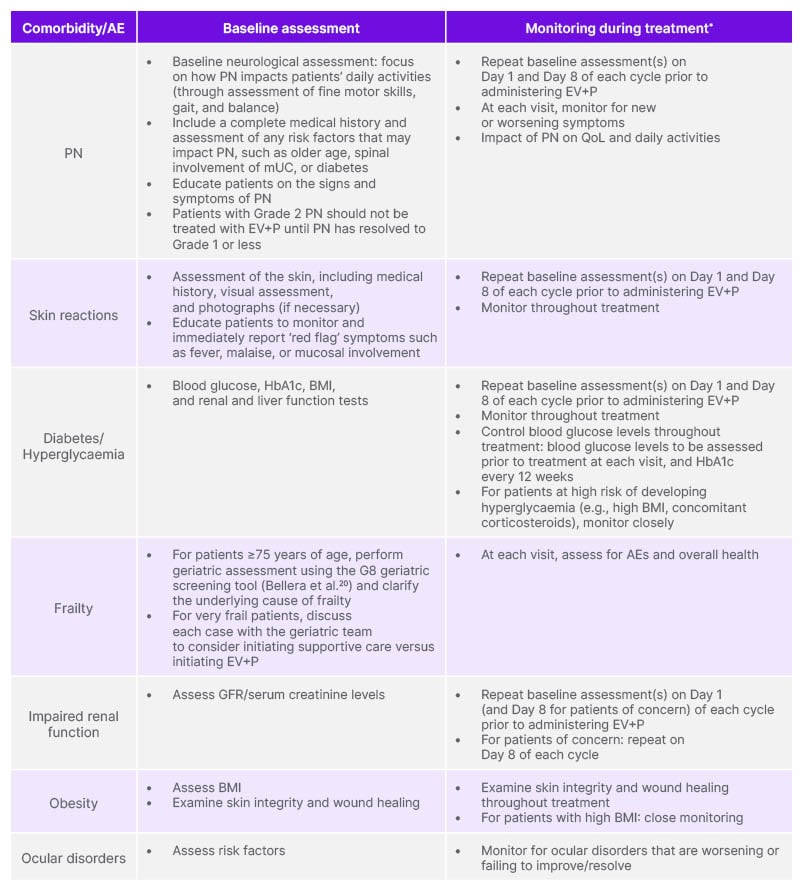
Table 3: Panel recommendations for monitoring patients with unresectable/metastatic urothelial cancer prior to and during treatment with enfortumab vedotin.
*Refer to the EV SmPC for detailed information on management.
AE: adverse event; EV: enfortumab vedotin; GFR: glomerular filtration rate; mUC: metastatic urothelial carcinoma; P: pembrolizumab; PN: peripheral neuropathy; QoL: quality of life; SmPC: Summary of Product Characteristics.
Summary
A baseline neurological assessment should be performed by the treating physician, with monitoring of the impact of PN on quality of life and daily activities at each visit. Patients may be reluctant to report PN due to concerns about treatment interruption or discontinuation. It is therefore important to educate patients on the signs and symptoms of PN, as well as potential management strategies, and to encourage prompt reporting of any relevant symptoms or neurological changes. Initiation of EV+P in patients with unresectable or mUC and baseline PN, or those at risk of developing PN, should be conducted in accordance with SmPC guidance. Patients with Grade ≥2 PN should not be treated with EV+P until PN has resolved to Grade 1 or less. SmPC recommendations should also guide management in the event of PN occurrence or worsening.
Patients with Unresectable or Metastatic Urothelial Carcinoma and Pre-Existing Skin Conditions, or Who May Be at Risk of Developing Skin Toxicities
Skin toxicities are recognised AEs associated with EV because of EV binding to Nectin-4 expressed in the skin.12 Although no established risk factors have been identified, a personal or family history of skin reactions or prior targeted therapies may predispose patients to skin toxicities.21
Mild-to-moderate skin reactions, predominantly maculopapular rash, have been reported with EV.12 In addition, severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), with fatal outcome have also occurred in patients treated with EV. In the pooled safety population of 564 patients who received EV+P in EV-302 and EV-103, any-grade skin reactions occurred in 70% of patients, and Grade 3 or 4 skin reactions occurred in 17% of patients. The median time to onset of severe skin reactions was 1.7 months (range: 0.1–17.2), and maculopapular rash led to discontinuation in 2% of patients. Of those patients who experienced a skin reaction of any grade, for whom data regarding resolution were available (n=391), 59% had complete resolution at last follow-up. Of the patients with residual skin reactions at last follow-up, 27% (n=43/159) had Grade ≥2 skin reactions.12
Treatment with EV+P should be initiated per the SmPC guidance. Patients should be monitored for the first signs of severe skin reactions, starting with the first cycle and throughout treatment, following the SmPC guidance. Management of mild-to-moderate skin reactions may include topical steroids and antihistamines. Fever or flu-like symptoms may be the first sign of a severe skin reaction; if this occurs, patients should be monitored closely and treatment withheld. For suspected SJS or TEN, or in case of bullous lesions onset, treatment should be withheld immediately and patients referred to specialised care. Histological confirmation, including performing multiple biopsies, is critical to early recognition, as timely diagnosis and intervention can improve prognosis. In cases of confirmed SJS or TEN, or Grade 4 or recurrent Grade 3 skin reaction, permanently discontinue EV. In patients with a worsening Grade 2 skin AE, a Grade 2 with fever skin reaction, or their first Grade 3 skin reaction, treatment should be withheld until reduction to Grade ≤1; see Table 2 and the EV SmPC for further guidance on dose modifications.12
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and baseline peripheral skin rash or pre-existing skin conditions
The panel advised that, irrespective of pre-existing skin conditions, an assessment of the skin, including medical history, visual assessment, and photographs (if necessary), should be performed prior to treatment initiation. It should be noted that records of skin health are particularly important for patients who do not have a partner or carer who can help with observing skin changes. The panel highlighted that patients with a pre-existing skin condition should be closely monitored when initiating EV+P.
Severe skin reactions predominantly occur during the first cycle of treatment with EV; therefore, monitoring should be performed at the first injection and throughout treatment.12 Patients should be educated to pay particular attention to any ‘red flag’ symptoms, such as fever, malaise, or mucosal involvement. Painful sores or ulcers in the mouth, nose, throat, or genital area; skin blistering or peeling; swollen lymph nodes; rash or itching that continues to get worse or comes back after treatment; or flu-like symptoms must be reported to an HCP immediately, as they may be an early indication of severe skin reactions, which can be fatal.12,15
Treatment with EV+P should be initiated per the SmPC guidance, and the presence of well-controlled, mild skin conditions not associated with any red flag symptoms listed above should not delay treatment initiation. Skin reactions that occur throughout treatment should be managed per the SmPC guidance, and a dermatologist may be consulted at baseline or during any cycle, as recommended by the SmPC.12
Summary
Treatment should be initiated per EV SmPC guidance, and patients should be monitored from the first and throughout subsequent treatment cycles. Mild-to-moderate skin reactions, predominantly maculopapular rash, have been reported with EV. In addition, severe cutaneous adverse reactions, including SJS and TEN, with fatal outcome have also occurred in patients treated with EV.12 Patients should be educated to recognise and report early symptoms of severe skin reactions to an HCP immediately, as these can be fatal.12,15 Skin reactions should be managed per SmPC guidance.
Patients with Unresectable or Metastatic Urothelial Carcinoma and Diabetes/Hyperglycaemia
Hyperglycaemia is an AE associated with EV+P and also with cisplatin when it is co-administered with high-dose corticosteroids.8,22 Risk factors for treatment-emergent hyperglycaemia include pre-existing diabetes/hyperglycaemia, a BMI of ≥30 kg/m2, illness/infection, the use of systemic steroids, or fatty liver disease.15 Poor glycaemic control may negatively affect treatment outcomes in patients with bladder cancer.23 Clinical experience from literature suggests that where blood sugar management can be instituted effectively, it should not prevent treatment with EV+P, and fluctuations may be easier to manage compared with those observed in patients receiving high-dose steroids administered concurrently with cisplatin chemotherapy.22
In the EV-302 trial, patients were required to have a verified blood glucose of <250 mg/dL prior to dosing, and patients with uncontrolled diabetes (defined as HbA1c ≥8%) were excluded from the trial.8 In the EV+P arm, hyperglycaemia of any grade was observed in 13.0% of patients, 6.1% of whom had Grade ≥3 hyperglycaemia.8 Hyperglycaemia and diabetic ketoacidosis, including fatal events, have been reported in patients with and without pre-existing diabetes treated with EV.12 Following initiation of EV+P, the median time to onset of Grade ≥2 hyperglycaemia was 0.5 months (range: 0.3–3.5).15
The EV SmPC states that, if blood glucose levels exceed 13.9 mmol/L (>250 mg/dL), EV is to be withheld until levels have reduced to ≤13.9 mmol/L (≤250 mg/dL); EV can then be resumed at the same dose level. For patients with blood glucose levels of ≤13.9 mmol/L (≤250 mg/dL), no changes to the initial EV dose are required.12
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and diabetes/hyperglycaemia
The panel advised that with appropriate management, diabetes and hyperglycaemia should neither preclude the initiation of EV+P nor cause unnecessary treatment delays. Baseline assessments should include blood glucose, HbA1c, BMI, and periodic renal and liver function tests throughout treatment. Blood glucose should be monitored at every treatment visit, and HbA1c should be monitored every 12 weeks. If blood glucose exceeds 13.9 mmol/L (>250 mg/dL), treatment should be temporarily withheld until levels decrease to ≤13.9 mmol/L, at which point EV+P can be resumed at the same dose.12 Patients at high risk of hyperglycaemia (such as those with elevated BMIs or concurrent corticosteroid use) require close monitoring. Education is key; patients should be counselled on the symptoms of hyperglycaemia and the importance of timely reporting.15
Summary
Diabetes and hyperglycaemia should not preclude the initiation of EV+P or cause unnecessary treatment delays, provided that these conditions are appropriately managed. Baseline assessments should include blood glucose, HbA1c, BMI, and renal and liver function tests. The panel advises monitoring blood glucose at each treatment visit, HbA1c every 12 weeks, and renal and liver function periodically throughout treatment. In the event of elevated blood glucose levels, guidance detailed in the EV SmPC regarding dose modifications should be followed.12
Patients with Unresectable or Metastatic Urothelial Carcinoma Who Are Frail or Unfit
Frailty is common among patients with bladder cancer, with almost half the patients considered frail or prefrail at diagnosis and treated with radical cystectomy.24
In the EV-302 trial, patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤2, and the median age was 69 years in both arms.8 Outcomes were improved in both the ECOG PS 0 and 1/2 subgroups in patients who received EV+P versus PBCT.8,9 The EV SmPC does not state any requirements for adjusting the EV dose based on patient fitness.12
Panel response on treating patients with unresectable or metastatic urothelial carcinoma who are frail/unfit
The panel advised that assessment of frailty is important before initiating any treatment for any disease and emphasised the importance of understanding if the frailty is due to the tumour or due to comorbidities. A geriatric assessment at baseline is recommended for patients ≥75 years of age, using the G8 geriatric screening tool,20 with monitoring for AEs and overall health at each visit. For very frail patients, initiating supportive care alone versus initiating EV+P plus supportive care should be discussed among the geriatric team on a case-by-case basis.
The SmPC does not state any requirements for adjusting the EV initial dose based on patient fitness.12
Summary
Frail patients should be assessed on an individual basis prior to treatment initiation. Clarifying the underlying cause of frailty (whether frailty is tumour-related or due to comorbidities) is of central importance. There are no specific starting dose modifications for EV in this patient population. Prescribers should initiate EV at the full recommended dose, with subsequent adjustments made on an individual basis according to the patient’s clinical context.
Patients with Unresectable or Metastatic Urothelial Carcinoma and Impaired Renal Function
EV is metabolised by the cytochrome P450 3A4 (CYP3A4) liver enzyme, and monomethyl auristatin E (MMAE; the protease-cleavable payload of EV) is excreted via faeces and urine.1 Population pharmacokinetic analysis of the renal insufficiency cohort (creatinine clearance ≥15 mL/min and <30 mL/min) included in the EV-101 study demonstrated that participants with severe renal impairment have the same benefit–risk ratio as patients with normal renal function; there was no significant difference in exposure of antibody–drug conjugate and unconjugated MMAE in patients with mild, moderate, or severe renal impairment compared to those with normal renal function.25 Renal impairment does not seem to impact EV pharmacokinetics.12
Renal function, as part of the Galsky criteria, is used to assess cisplatin ineligibility (glomerular filtration rate [GFR] ≥60 mL/min to be eligible for cisplatin), and has guided 1L mUC treatment selection previously when PBCT was the standard of care.10 As the EV-302 trial compared outcomes and safety profiles in patients randomised to receive EV+P or PBCT, patients were required to be eligible to receive PBCT, per investigator’s judgement, and have a GFR ≥30 mL/min/1.73 m2 (patients with an ECOG PS of 2 were required to also meet the additional criteria: haemoglobin ≥10 g/dL and GFR ≥50 mL/min, but may not have New York Heart Association [NYHA] Class III heart failure).8 For patients who received EV+P, overall and progression-free survival were improved (hazard ratio: ≤0.5) versus PBCT in the normal, mild, and moderate/severe renal function subgroups.8,9
The EV SmPC states no contraindication or dose modifications are required for patients with mild (creatinine clearance [CrCl]: >60–90 mL/min), moderate (CrCl: 30–60 mL/min), or severe (CrCl: 15–<30 mL/min) renal impairment. EV has not been evaluated in patients with end-stage renal disease (CrCl: <15 mL/min).12
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and impaired renal function
Impaired renal function was not a cause of concern for the expert panel, who reinforced that EV is metabolised by the liver and does not seem to affect renal function.1,26 The panel stated that, generally, they felt comfortable prescribing EV+P to patients with mUC and impaired renal function, but with monitoring considerations as described below.
Advisors recommended assessing baseline renal function via a GFR or serum creatinine test, with tests to be repeated on Day 1 of each cycle prior to administering EV+P. For patients of concern, an additional monitoring step was suggested on Day 8 of every cycle.
Summary
The primary route of elimination for EV and MMAE is not renal, renal impairment does not seem to impact EV pharmacokinetics,12 and EV does not seem to affect renal function.1,26 For patients with impaired renal function, the recommendation is to assess renal function at baseline. At each cycle, patients should be monitored for any significant renal function changes before proceeding with EV+P treatment. For patients with mild, moderate, or severe renal impairment, no initial dose modifications of EV+P are required. EV has not been evaluated in patients with end-stage renal disease (CrCl: <15 mL/min).12
Patients with Unresectable or Metastatic Urothelial Carcinoma and Obesity
Obesity is a common comorbidity among patients with bladder cancer. In one UK study, 66% of patients with bladder cancer were reported as overweight or obese.27 BMI was not considered as part of the inclusion or exclusion criteria for patients in the EV-302 trial, and analyses were not performed in this population as BMI was not included as a pre-specified subgroup of interest.8 To note, hyperglycaemia has been reported to occur more frequently in patients with a BMI of ≥30 kg/m2, or with baseline hyperglycaemia or diabetes.12,15
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and obesity
The panel advised that baseline obesity should generally not exclude patients from, or delay, treatment with EV+P. Recommendations for patients who are obese were a baseline BMI assessment and close monitoring of those with high BMIs, as this is a known risk factor for developing hyperglycaemia.
For patients who are obese, advisors also recommended careful examination of skin integrity and wound healing, as it may be difficult to observe skin changes in areas such as skin folds.
Summary
Obesity should not preclude patients from or delay treatment with EV+P. For patients ≥100 kg, the maximum EV dose per infusion is 125 mg.12
Patients with Unresectable or Metastatic Urothelial Carcinoma and Ocular Disorders
Ocular disorders have been reported in patients treated with EV+P.12 In the EV-302 trial, 21.4% of patients treated with EV+P experienced any-grade ocular disorders. The most common ocular disorder reported was dry eye, which occurred in 18.6% of patients. No ocular disorders of Grade ≥3 were reported with EV+P.8 Risk factors for ocular disorders include older age and contact lens use, and patients should be monitored for the occurrence of ocular disorders.12,15
Panel response on treating patients with unresectable or metastatic urothelial carcinoma and the occurrence or risk of occurrence for ocular disorders
The panel advised that patients should be monitored for ocular disorders; however, a full assessment at baseline was generally considered unnecessary. Treatment with EV+P should be initiated per the SmPC guidance, and prophylaxis of symptoms should be considered, e.g., artificial tears to prevent dry eye. Patients should be monitored for the occurrence or worsening of any ocular disorder, which should be managed per the SmPC guidance, with referral for ophthalmological evaluation if symptoms fail to resolve or worsen.12
Summary
Ocular disorders should be monitored and managed per the SmPC guidance, and patients should be referred for ophthalmological assessment if ocular disorders fail to resolve or worsen.12
A summary of panel recommendations for all pre-existing comorbidities and AEs discussed in this article, supported by SmPC guidance, is given in Table 3.
DISCUSSION
EV+P is the preferred 1L regimen for patients with unresectable mUC who meet its approved indication, as reflected in multiple treatment guidelines.5,7,12 This article, therefore, seeks to provide practical recommendations to support the use of EV+P in patients with clinically complex profiles, based on SmPC guidance, expert insights, and available clinical data.
The panel advised that for patients with multiple comorbidities, the most urgent clinical need is usually to treat the cancer itself. Delaying treatment to manage patients’ comorbidities must be balanced against the potential for disease progression and the loss of opportunity for early cancer control.
As AEs may occur during treatment with EV+P, it is crucial to recognise, monitor, and manage them effectively. This approach may enable patients to remain on treatment to help achieve the desired outcomes and minimise the risk of premature discontinuation. Dose modifications are expected over time, resulting in a personalised treatment course for each patient. Such dose modifications are not only acceptable but are also often necessary to enhance patient care, in line with standard clinical practice.
For patients who are frail and/or have baseline comorbidities, prescribers should refer to the SmPC recommendations, which support initiating EV at the full, approved dose.12
The post hoc analysis of EV monotherapy in EV-101 showed that patients who received the recommended starting dose of EV had a greater overall response rate versus patients who initiated treatment with lower starting doses.18 In addition, in EV-201 Cohort 1, responding patients resumed treatment and continued to benefit following dose modifications.18
Moreover, the exploratory analysis of EV-302 showed that appropriate treatment interruptions allowed for responders to continue treatment, with a safety profile similar to that in the overall population, despite receiving more cycles of therapy versus the PBCT arm.19
Lastly, in the UNITE retrospective study, patients with baseline neuropathy and/or diabetes who received EV monotherapy or EV+P (N=666, all patients; 13% of patients received EV+P in 1L) had similar outcomes to patients without such comorbidities.28
As EV is approved for 1L treatment in combination with pembrolizumab, some AEs, such as skin toxicities, are associated with both pembrolizumab and EV;12,14 AEs related to pembrolizumab are not discussed in this article. For details on AEs relating to pembrolizumab and their suggested management, please refer to the pembrolizumab SmPC.14
CONCLUSION
EV+P is the standard-of-care 1L treatment for patients with unresectable or mUC who are eligible for PBCT, and patients who meet its approved indication should be given the opportunity to receive this regimen without unnecessary clinical restrictions. Appropriate clinical assessment at treatment initiation is important to ensure that comorbidities and frailty are adequately considered in patient care. Best practice is to initiate EV+P at the recommended starting dose, per the respective SmPCs, with appropriate dose interruption, reduction or discontinuation applied when clinically required, including for patients with comorbidities or frailty. Clinical judgment and open dialogue are crucial to ensure that each patient receives the most appropriate and effective treatment.
Click here for the UK prescribing information
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018. |
For HCPs located in Europe, please refer to the EMA SmPC for PADCEV™ (enfortumab vedotin) via the following link: https://www.ema.europa.eu/en/documents/product-information/padcev-epar-product-information_en.pdf
This link will take you to a non-Astellas website. Astellas does not endorse or accept liability for sites controlled by third parties.