The publication of this infographic was funded by Boehringer Ingelheim. This content is intended for US Healthcare Professionals.
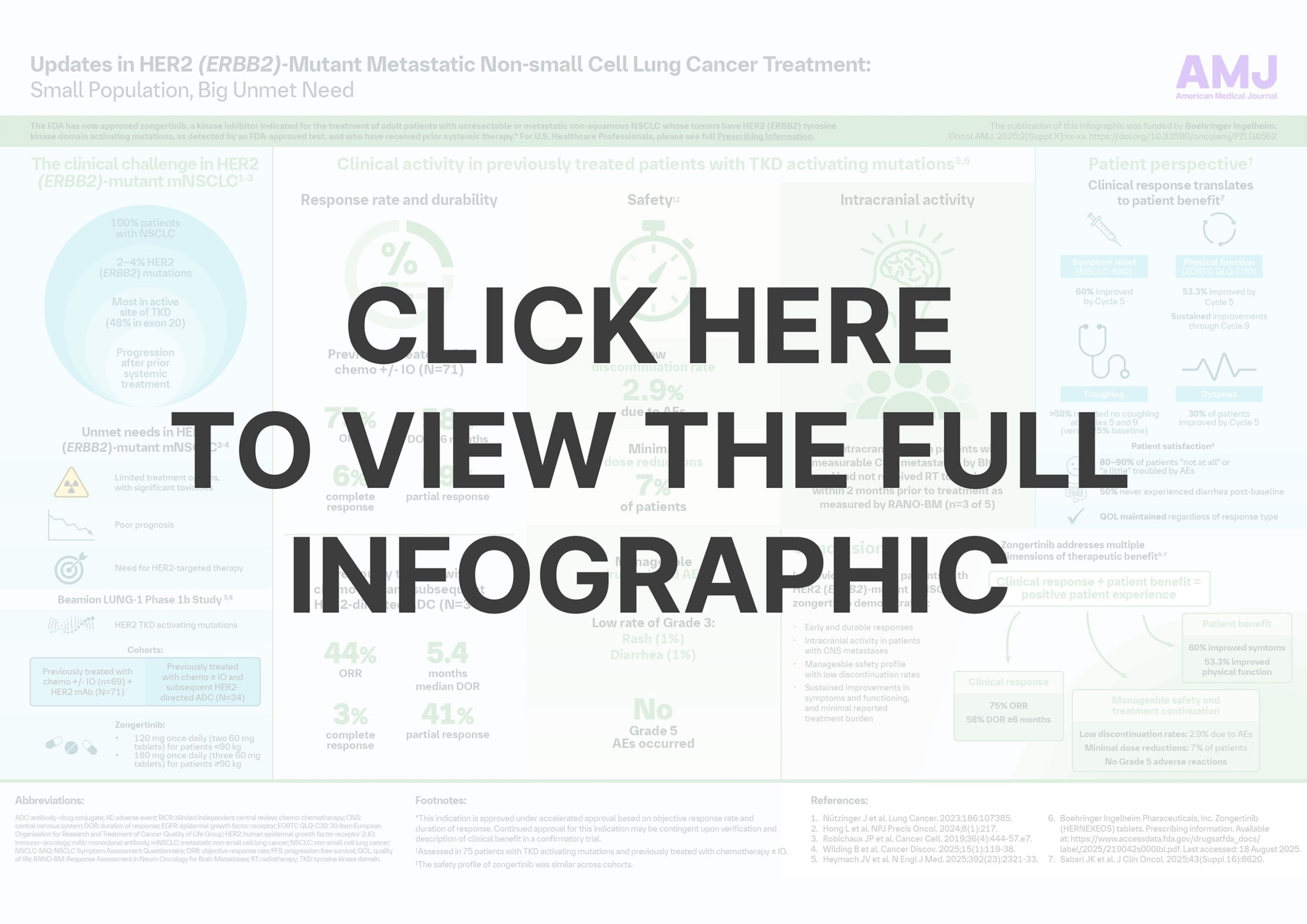
HER2 (ERBB2)-mutant metastatic non-small cell lung cancer (NSCLC) remains a clinical challenge, with limited treatment options and poor prognosis. The FDA has now approved zongertinib, a kinase inhibitor indicated for the treatment of adult patients with unresectable or metastatic non-squamous NSCLC whose tumors have HER2 (ERBB2) tyrosine kinase domain activating mutations, as detected by an FDA-approved test, and who have received prior systemic therapy.*
This infographic explores the clinical evidence and how this new therapeutic option may be considered in clinical practice.
Explore:
- Evidence of clinical responses over time
- Findings related to intracranial activity in patients with CNS metastases
- Insights on safety and treatment continuation
- Patient perspectives on symptoms, functioning, and treatment experience
*This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. For U.S. Healthcare Professionals, please see full Prescribing Information.