Meeting Summary
Infections are the second leading cause of non-cancer-related mortality in the first year of life after a patient is diagnosed with cancer. Vaccination is a key aspect of cancer care to reduce infection risk and the severity of infection-related complications, with the intent of enhancing cancer-related outcomes and quality of life. This article summarizes a GSK-sponsored industry theater, “Immunize to Optimize: Enhancing Cancer Care Through Vaccines”, held as part of the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, USA, from May 30th–June 3rd 2025. The industry theater included presentations from three speakers from GSK in the USA: Leonard Friedland, Noha Eltoukhy, and Jean A. Hurteau. Friedland described how vaccination is crucial in reducing infection risk for patients with cancer, and that prioritizing vaccination strategy is key to comprehensive cancer care. Eltoukhy discussed the ASCO guideline for vaccination of adults with cancer, including key vaccinations recommended for this population and special considerations in adults with hematological cancer. Leading on from this, Friedland also considered ASCO’s key initiatives to improve vaccination rates in high-risk patients, and the importance of building a “wall of protection” to safeguard patients with cancer through vaccination. Hurteau presented two patient case studies, one for a patient with a solid malignancy and the other for a patient with a hematological malignancy, setting the scene for discussion about vaccination strategy for patients with cancer. These patient cases provided a forum to consolidate the information learned about the ASCO guideline and discuss evidence-based approaches to enhancing cancer care through vaccination, including best practices and timing of vaccinations for patients with cancer. This article presents the key takeaways from the industry theater and reinforces the importance of proactive vaccination in cancer care, underscoring vaccination as an important, evidence-based component of oncology management to reduce infection-related complications and potentially improve patient outcomes.
Introduction
Leonard Friedland
Vaccination is Crucial in Reducing Infection Risk for Patients with Cancer
Patients with cancer often have a compromised immune system, which places them at an increased risk of infection that can extend beyond active cancer treatment.1 Infections are the second leading cause of non-cancer-related mortality, after cardiovascular mortality, in the first year following a cancer diagnosis,2 with most infection-related deaths attributed to influenza and pneumonia.1 Rates of fatal infection among patients with cancer are reported to be nearly three times higher than in the general population.3 Friedland acknowledged that the management of patients with cancer who have a compromised immune system is extremely challenging.
The goal of vaccination is to protect the recipient from infection when possible, and to limit the severity of disease when the infection cannot be completely prevented.1 Effective vaccination can reduce the severity of infectious disease, the occurrence of infection-related complications, and the associated hospitalizations in patients with cancer; therefore, prioritizing vaccination is likely to enhance cancer-related outcomes and quality of life.1 Friedland acknowledged that a cancer diagnosis can be overwhelming for the patient, and vaccination may not be an immediate priority for the physician to incorporate into the care plan. He also emphasized that proactive infection prevention and vaccination discussions are essential elements of comprehensive cancer care, and a vaccination strategy should be considered vital in the overall care of patients with cancer.1
Empowering Vaccination Recommendations
In a prospective, cross-sectional survey trial conducted from March–November 2022 at a supportive care center at the MD Anderson Cancer Center, Houston, Texas, USA (n=100), 82% of patients with advanced cancer reported their physician was the most important source of information around vaccination decision-making.4 Furthermore, in a cross-sectional survey conducted at a medical center in Italy in February and March 2024 (n=128), approximately 50% of patients with solid tumors cited lack of an oncologist recommendation for vaccination, in the context of their cancer care, as a main reason for low vaccine uptake.5
Receiving a recommendation from a healthcare provider strongly predicts whether an individual will agree to be vaccinated.6-8 Friedland pointed out that vaccine acceptance among patients with cancer is influenced by their relationship with the oncologist and the oncology care team; therefore, interactions between oncologists and patients, as well as vaccine counseling by the oncology care team, are critical to optimize vaccination uptake.
Integrating Vaccination Conversations with Patients is Pivotal During Routine Oncology Practice
Oncologists are a critical resource for patients with cancer regarding vaccine safety and efficacy before, during, and after cancer treatment.1 Documenting the patient’s vaccination status at the first clinic visit, and timely provision of recommended vaccines thereafter, are crucial first steps in cancer management.1 Integrating conversations on vaccination and vaccine recommendations with patients is pivotal during routine oncology practice. Furthermore, partnering with primary care providers, nurses, and pharmacists will enhance the collective ability to ensure comprehensive vaccination.1
Friedland advocated for healthcare providers caring for patients with cancer to make vaccination a routine component of every care plan, and underscored that actively addressing vaccination empowers the patient with a vital layer of protection, thereby directly contributing to better health outcomes.
Review of the ASCO Guideline
Noha Eltoukhy
ASCO Guideline Recommendations
The purpose of the ASCO guideline is to guide vaccination strategies for adults with solid tumors or hematological malignancies, including those undergoing intensive therapies and long-term survivors.1 The guideline recommends that clinicians should determine vaccination status at the time of cancer diagnosis, and ensure that adults newly diagnosed with cancer and about to start treatment are up to date on seasonal vaccines and age- and risk-based vaccines.1 Furthermore, vaccination should ideally precede any planned cancer treatment by 2–4 weeks; however, non-live vaccines are suitable for administration during or after chemotherapy or immunotherapy, hormonal treatment, radiation, or surgery.1
Eltoukhy stated that optimizing vaccination is a key element in the care of patients with cancer to reduce the risk and severity of infections, and that making vaccinations a routine component of every care plan in these patients is vital for improving outcomes.
Key Vaccinations Recommended for Adults with Cancer
The routine vaccinations (all being non-live vaccines) recommended for adults with cancer are shown in Figure 1A.1 Eltoukhy noted that the CDC pneumococcal vaccination recommendations include the conjugate vaccine PCV21 as well as PCV20,9 and the former may be added to the ASCO guideline in the future, along with other updates that occur to the CDC immunization schedule.
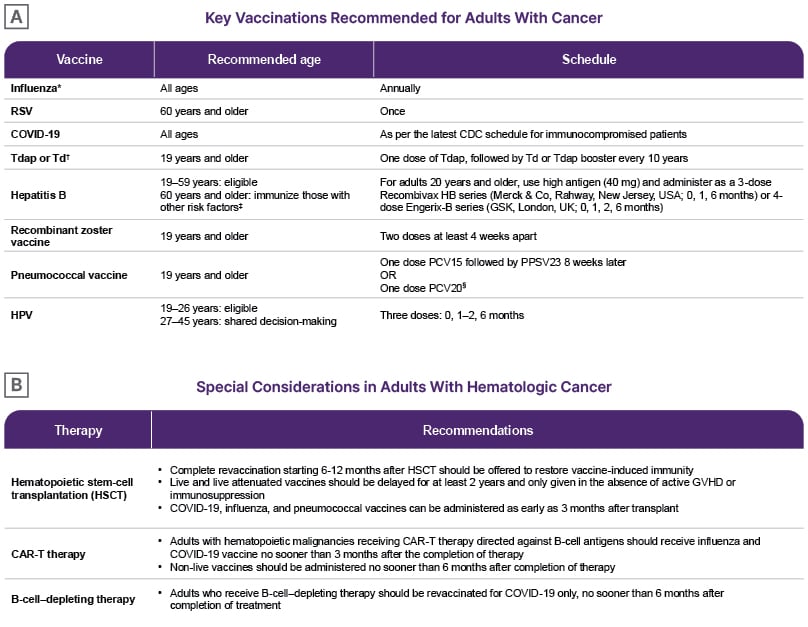
Figure 1: American Society of Clinical Oncology guideline recommendations.
Adapted from Kamboj M et al.1
NOTE: Adapted from CDC Adult Immunization Schedule by Medical Condition and Other Indication. Information linking to US trade names for each vaccine is available and routinely updated at the CDC’s website on vaccines. Coadministration of two or more of the recommended non-live vaccines is acceptable per CDC guidelines. When given on separate days, there is no recommended waiting period. Note, PCV15 and PPSV23 should be separated by at least 8 weeks as noted in the table.
*Live attenuated influenza vaccine, which is administered as a nasal spray, cannot be given to patients with cancer.
† Tdap has lower amounts of diphtheria and pertussis toxoid, and is only used for those 7 years and older. DTaP, the
pediatric vaccine for prevention of tetanus, diphtheria, and pertussis, is only for children younger than 7 years.
‡HIV, chronic liver disease, intravenous drug use, sexual risk factors, incarcerated individuals.
§Patients who have previously received PCV13 only can receive one dose of PCV20 after an interval of 1 year.
HPV: human papillomavirus; PCV: pneumococcal conjugate vaccine; PPSV23: 23 valent pneumococcal polysaccharide vaccine; RSV: respiratory syncytial virus; Td: tetanus and diphtheria; Tdap: tetanus, diphtheria, and pertussis.
Special Considerations in Adults with Hematological Cancer
Additional considerations surrounding the timing of vaccination and the need to revaccinate for adults with hematological cancers, based on the degree of immunosuppression and the type of therapy, are shown in Figure 1B.1 Eltoukhy highlighted that, per the ASCO guideline, long-term survivors of hematological malignancy, with or without active disease, and individuals with long-standing B cell dysfunction or hypogammaglobulinemia from therapy or B cell lineage malignancies, should receive the recommended non-live vaccines, even though their response to the vaccines may be attenuated.
Vaccine Recommendations for Travel
Adults with solid or hematological malignancies traveling to an area of risk should follow the CDC standard recommendations for the destination.1 The CDC Yellow Book recommends that travel vaccinations for patients with solid malignancies should generally be delayed until at least 3 months from last chemotherapy exposure, and with disease in remission.10 In addition, recipients of hematopoietic stem cell transplant ideally should delay travel for >2 years after transplant to allow for full revaccination.10
Vaccine Recommendations for Household Members and Close Contacts
All household members and close contacts of patients with cancer are recommended to keep up to date with vaccinations, where feasible.1 Live attenuated influenza vaccine is not recommended for individuals in close contact with patients who have recently received hematopoietic stem cell transplant or have graft-versus-host disease.1 In addition, oral poliovirus and smallpox vaccines should not be given to family members of immunocompromised individuals.1
A Multidisciplinary Care Team Approach to Vaccination
A multidisciplinary care team approach to vaccination involving primary care providers, nurses, pharmacists, and oncologists is recommended,1,11 although Eltoukhy pointed out that not all patients have a primary care provider. Furthermore, provider endorsement and patient education are essential to overcome vaccine hesitancy.1 Eltoukhy remarked, “We always look at vaccination as a team sport. The more support from all angles of the care team there is, the more successful the team will be in having vaccine conversations with patients and encouraging them to stay up to date with the recommended vaccines for their optimal protection.”
Barriers to Implementing Vaccination in Routine Care
To conclude this presentation, Eltoukhy asked the audience to define the biggest barriers to implementing vaccinations into routine cancer care treatment plans. The audience was invited to provide free text and the results were presented in a word cloud. By far, the most common barrier was awareness, with other examples including education, availability, ignorance, fear, accurate medical history, lack of time or staff, reimbursement, and mindset. Eltoukhy emphasized that initiating vaccine conversations with patients and making recommendations for vaccination is a good starting point to overcome these barriers.
ASCO Initiatives
Leonard Friedland
ASCO’s Key Initiative to Improve Vaccination Rates in High-Risk Patients
ASCO received funding under a 5-year cooperative agreement between the CDC and the Council of Medical Specialty Societies (CMSS) for a project to improve rates of recommended vaccinations for adults with cancer and blood disorders.12 Since 2023, ASCO has been collaborating with eight oncology health systems across the USA to implement the Specialty Societies Advancing Adult Immunization (SSAAI) Project.12 The objective is to develop, test, and measure strategies to inform decisions about immunization practices and improve vaccination rates among high-risk adults, including patients with cancer, across the USA.12 This initiative is part of ASCO’s broader efforts to improve vaccination rates in vulnerable populations.
Friedland explained that the overall aim of the SSAAI project is to incorporate the CDC Standards for Adult Immunization practice into clinical care, and drive immunization through outreach, education, and quality improvement efforts.12 Friedland described this project as part of the “really fantastic” work that is happening at ASCO for the comprehensive care of patients and infection prevention, and highlighted how oncologists can be vaccine champions for their patients.
Central Arkansas Radiation Therapy Institute Initiative for Improving Vaccination Rates
Friedland then focused on the outcomes from one of the eight centers in the SSAAI project, the Central Arkansas Radiation Therapy Institute (CARTI) in Little Rock, Arkansas, USA.13 ASCO reviewed the outreach, education, and assessments at CARTI, with the goal of boosting vaccinations in patients with cancer at the institute. Various stakeholders at CARTI, including physicians, nurses, pharmacists, IT specialists, and the leadership team, contributed to the launch and organization of the SSAAI project.
Implementation strategies included identifying early adopters of COVID-19 vaccination strategies in the center and positioning them as vaccine champions, leveraging the trust between healthcare professionals and patients to address vaccine hesitancy, and integrating vaccination into oncology workflows and patient visits.13
The focus on vaccination and infectious diseases prevention at the center improved vaccination rates among high-risk patients with cancer, with 82% of patients preferring to receive all required vaccines (not just influenza and COVID-19 vaccines). The nursing staff had a pivotal role in this success.13 Friedland commented, “CARTI’s commitment to engage with patients about the importance of vaccinations has led to record immunization rates, which shows that patients trust their providers.”
Building a “Wall of Protection” to Safeguard Patients with Cancer Through Vaccination
Building a “wall of protection” to safeguard patients with cancer through vaccination is a multifaceted approach to reduce infection-related morbidity and mortality, and improve outcomes and quality of life (Figure 2).1 Friedland stated, “Open communication between the oncology care team and the patient is critical, particularly in today’s environment where trust in vaccines is under pressure. Strategies to address the unique challenges that contribute to low vaccination rates in patients with cancer are essential.”Friedland considered that effective vaccination protocols are best integrated into the clinic workflow plans, and there is a need to develop and study vaccines that are more immunogenic in individuals who are immunocompromised. The participation of patients who are immunocompromised in clinical trials will contribute to the understanding and improvement of therapeutics and preventative options. Friedland concluded that vaccines should be included as a critical component in comprehensive patient care for patients with cancer.
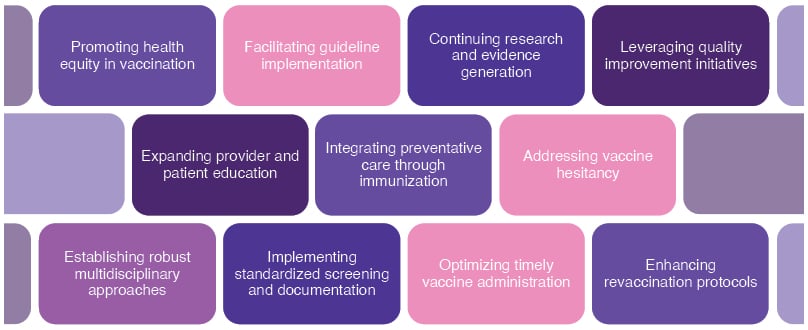
Figure 2: Building a wall of protection to safeguard patients with cancer through vaccination.
Patient Case Studies
Jean A. Hurteau
Hurteau presented two patient case studies, one for a patient with a solid malignancy, and the other for a patient with a hematological malignancy. These case studies provided a forum for discussion of the ASCO guideline, focusing on evidence-based approaches to enhancing cancer care through vaccination, as well as best practices and timing of vaccinations for patients with cancer.
Case Study 1: A Patient with a Solid Malignancy (Ovarian Cancer)
Claire, a 62-year-old female, presented with a distended abdomen (bloating), fatigue, and decreased appetite, had a medical history of hypertension, and was taking hydrochlorothiazide (a diuretic) (Figure 3A). Claire’s vaccine history was up to date, except for influenza and COVID-19 vaccinations. She had not undergone surgery and had no allergies. Her mother died of breast cancer at age 45 years.

Figure 3: Patient case studies.
NOTE: These patient cases are fictional representations of relevant patient profiles for discussion purposes
BP: blood pressure; CA: cancer antigen; C/A/P: chest/abdomen/pelvis; CBC: complete blood count; CD: cluster of differentiation; FH: family history; FISH: fluorescence in situ hybridization; HTN: hypertension; HZ: herpes zoster; IPPA: inspection, palpation, percussion, and auscultation; P: pulse; PCA: patient-controlled analgesia; PMH: past medical history; PSH: past surgical history; R-ISS: Revised International Staging System; RR: respiratory rate; RV: rectovaginal; SUVmax: maximum standardized uptake value.
Abdominal examination revealed fluid wave and nodularity, likely consistent with a floating omental cake (an abnormally thickened greater omentum). Pelvic and rectovaginal examination also revealed nodularity. The workup included a CT scan of the chest, abdomen, and pelvis, which revealed small pleural effusion, ascites, and omental cake. The cancer antigen 125 (CA125) level was elevated. All these findings are consistent with advanced ovarian cancer.
Hurteau explained that the usual approach for patients with advanced ovarian cancer is to perform cytoreductive surgery. This necessitates several patient visits to discuss the care plan, the risks, potential complications, expectations of surgery, the likely duration of hospitalization, and the management of the patient after surgery, including chemotherapy and maintenance therapy.
Claire underwent cytoreductive surgery, and a diagnosis of Stage IIIC epithelial ovarian cancer was confirmed. She recovered in hospital and then went home. Chemotherapy was started 4 weeks after surgery. During the second cycle of chemotherapy, at Day 20, Claire developed fever and chills, a sore throat, cough, and runny nose.
The workup included a complete blood count, which showed no neutropenia. Claire tested positive for COVID-19, and her induction chemotherapy had to be delayed until she recovered from this infection.
The audience was asked to consider when the indicated vaccines should have been administered to Claire. All respondents voted for “before starting a treatment plan.” There were no votes for “during treatment, if feasible,” “after completing therapy,” or “timing does not matter.” Hurteau confirmed that the best time to administer the indicated vaccines was as early as possible, in accordance with the ASCO guideline.1 According to the audience, primary care providers, along with oncologists, should be primarily responsible for collecting vaccine history and administering vaccines to patients with cancer. However, Hurteau pointed out that many of these patients are referred through the emergency room or have no primary care provider; therefore, other frontline healthcare providers need to take responsibility.
Hurteau identified that Claire had attended several visits to physicians and had been hospitalized, providing multiple opportunities for vaccines to be administered prophylactically. The CDC recommends that the COVID-19 vaccine should be administered 3 months after either the date symptoms started or the date of a positive COVID-19 test in asymptomatic patients,14 which Hurteau commented likely aligns with the end of Claire’s treatment, and this timing was acceptable. Hurteau noted that there are many opportunities to administer the influenza vaccine, such as Day 1 or 5 of the chemotherapy cycle, or after mid-cycle, and it can be co-administered with the COVID-19 vaccine.
Case Study 2: A Patient with a Hematological Malignancy (Multiple Myeloma)
Damien, a 55-year-old male, presented with thoracic back pain, with a medical history of myocardial infarction, which was treated medically, and was taking aspirin and ibuprofen (Figure 3B). Damien’s vaccine history was up to date for influenza and COVID-19 vaccinations, but he had not received pneumococcal or herpes zoster (HZ; shingles) vaccines. Damien had not undergone surgery and had no allergies. His paternal aunt had multiple myeloma.
The workup included a whole-body PET/CT scan, which showed a lytic lesion at the T8 vertebra. A bone marrow biopsy identified 60% involvement of the bone marrow by CD138 immunohistochemistry. Fluorescence in situ hybridization testing indicated a positive translocation t(11;14).
A diagnosis of revised international staging system (R-ISS) Stage I multiple myeloma was confirmed, and a care plan was developed. Damien was to undergo induction chemotherapy comprising six cycles of bortezomib, lenalidomide, dexamethasone, and daratumumab, followed by maintenance therapy with lenalidomide.
During the maintenance phase of the treatment, Damien experienced excruciating thoracic pain along dermatomes of the torso, with noted characteristic lesions of HZ crossing the midline, suggestive of a disseminated HZ infection. Damien was admitted to the hospital for pain control and management of HZ infection as an immunocompromised patient, and his maintenance therapy was held. He received intravenous hydromorphone patient-controlled analgesia for pain control and high-dose intravenous acyclovir for HZ. After 10 days in hospital, Damien’s pain was under control, the HZ lesions were healing, and he was discharged.
Hurteau specified that once Damien had recovered from acute illness, he should receive the HZ vaccine, as per current recommendations. He should also receive the pneumococcal vaccine, and then resume maintenance cancer treatment.
Avoiding Potential Patient Complications in Cancer Care
Hurteau summarized that ensuring patients with cancer are up to date with vaccinations, and administering these vaccinations as early as possible during the treatment course, are important preventive measures. The goal of these measures is to reduce the risk and severity of infectious disease and associated complications, avoid delays in cancer treatments due to infectious illness, and improve patient outcomes.
Considering the Vaccination Needs of Patients who Require Cancer Treatment
Hurteau asked the audience to specify when, in clinical practice, they consider the vaccination needs of patients who require cancer treatments. Most of the respondents consider the vaccination needs of these patients “at the time of initial cancer diagnosis,” with the remaining responses split equally between “immediately before initiating therapy” and “after completion of cancer treatment.” None of the audience responded with “during individualized treatment, if needed” or “I do not consider the vaccination needs.”
What Can Oncologists Do to Improve Vaccination Rates in Patients with Cancer?
Hurteau described oncologists as being “on the frontline of care for a uniquely vulnerable patient population,” and ideally placed to educate patients and their families about the critical role of vaccination in preventing infections that could complicate the treatment course. Hurteau recommended that oncologists should proactively assess vaccination status at the initial patient visit and prioritize timely administration of recommended vaccines, whether it be before, during, or after treatment;1 clearly communicate the importance of vaccination in the oncology treatment plan; leverage a multidisciplinary team approach to develop a vaccination plan into everyday workflow; and collaborate actively with the broader healthcare team to ensure patients receive the recommended vaccinations.







