Meeting Summary
Extended follow-up data from the CheckMate 274 trial have further solidified adjuvant nivolumab’s position as a standard of care in high-risk muscle-invasive urothelial carcinoma (MIUC), potentially offering a pathway to improved outcomes. These findings were presented by Matthew Milowsky, a medical oncologist and clinical and translational researcher at the University of North Carolina School of Medicine, Chapel Hill, USA, at the 2025 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU). This Phase III study evaluated the efficacy of adjuvant nivolumab in patients with high-risk MIUC after radical surgery. The new analysis, focusing on the muscle-invasive bladder cancer (MIBC) subgroup, demonstrated sustained disease-free survival (DFS) benefits and favorable overall survival (OS) trends with nivolumab, regardless of prior treatment status.
Introduction
The management of MIBC, a subtype of MIUC, remains a significant clinical challenge, with 29–37% of patients experiencing disease recurrence following radical surgery.1,2 Although cisplatin-based neoadjuvant chemotherapy (NAC) has shown survival benefits in patients with MIBC,3,4 many people are ineligible for NAC or decline treatment.5 These clinical gaps underscore the need for effective adjuvant therapies to improve MIBC outcomes.
Immunotherapy has transformed the first-line treatment landscape of urothelial cancers.6 Nivolumab, a programmed death-1 inhibitor, received approval from the US FDA in 2021 for the adjuvant treatment of high-risk urothelial carcinoma following radical resection, based on survival benefits in the CheckMate 274 trial.7,8 The extended follow-up analysis of the CheckMate 274 trial addressed unresolved questions about the long-term efficacy of nivolumab in specific patient subgroups, including in patients with MIBC who did and did not receive prior NAC.9
In addition, although recurrence rates following radical surgery in patients with MIBC are high,1,2 approximately 66% of patients remain recurrence-free 10 years after radical cystectomy.10 This raises concerns regarding overtreatment of patients who are at low risk of recurrence. More data are needed to help clinicians make informed decisions regarding which patient subgroups need adjuvant therapy, and which subgroups are likely to benefit from the treatment.
A Phase III Trial of Adjuvant Nivolumab in Patients with High-Risk Muscle-Invasive Urothelial Carcinoma
CheckMate 274 was a Phase III, randomized, double-blind, multicenter study comparing adjuvant nivolumab with placebo in patients with high-risk MIUC; patients with MIBC represented 79% of the treatment and placebo arms (279/353 [79.0%] patients in the nivolumab group and 281/356 [78.9%] of patients in the placebo group).7 The trial enrolled patients who had undergone radical surgery within 120 days and were disease-free within 4 weeks of randomization.7 Eligible patients included those with pathologic T stage ypT2-ypT4a or ypN+ MIUC who received NAC, as well as those with pT3-pT4a or pN+ MIUC without prior NAC who were either ineligible for, or had declined, adjuvant cisplatin chemotherapy.7
Patients were randomized 1:1 to receive either nivolumab 240 mg every 2 weeks or placebo for up to 1 year.7 Patients were stratified based on several factors, including tumor PD-L1 status (≥1% and <1% or indeterminate), prior NAC, and nodal status.7 The primary endpoints were DFS in all randomized patients (intention-to-treat population) and in those with tumor PD-L1 expression ≥1%. OS was a secondary endpoint.7 The subgroup analysis presented by Milowsky included data from 560 patients, with a median follow-up of 36.1 months in the intention-to-treat population, and 34.5 months in the MIBC population.9,11
Adjuvant Nivolumab Prolongs Disease-Free Survival
Among all randomized patients with MIBC, the median DFS was 25.6 months for nivolumab versus 8.5 months for placebo, with a hazard ratio (HR) of 0.63 (95% CI: 0.51–0.78; Figure 1). This benefit was consistent regardless of prior treatment with NAC. In patients who received prior NAC, the median DFS was 19.6 months with nivolumab versus 8.3 months with placebo (HR: 0.58; 95% CI: 0.43–0.79; Figure 1). In patients without prior NAC, median DFS was 25.9 and 13.7 months with nivolumab and placebo, respectively (HR: 0.69; 95% CI: 0.50–0.94; Figure 1).9
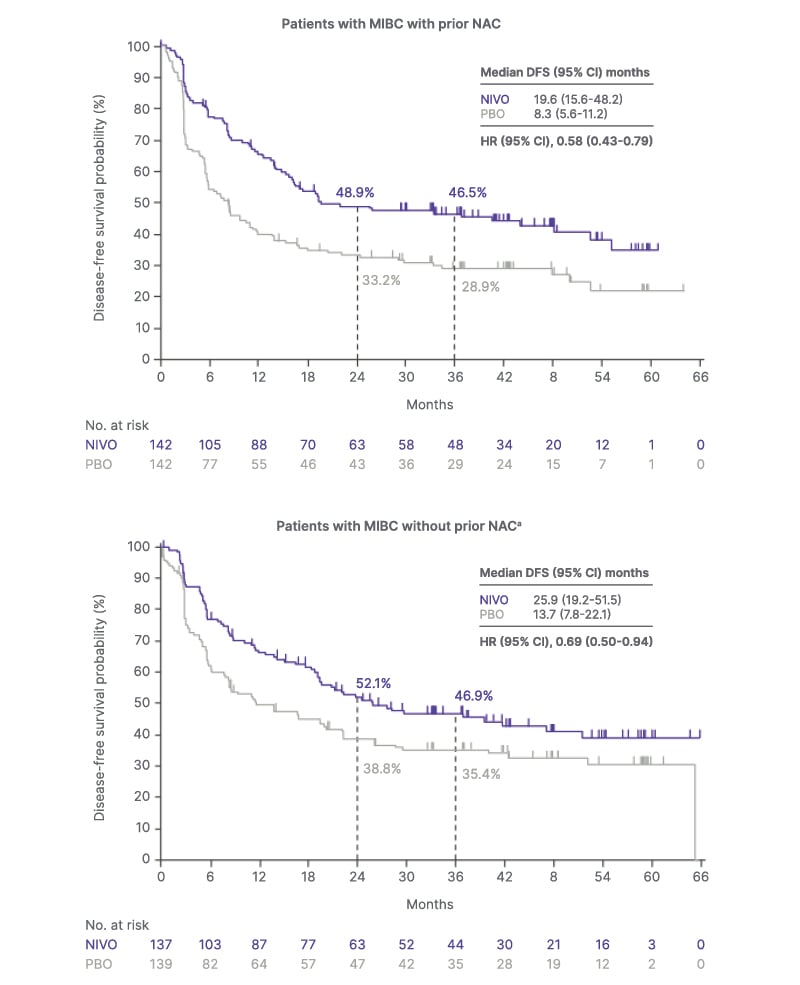
Figure 1: Kaplan-Meier curves showing disease-free survival in patients with muscle-invasive bladder cancer stratified by prior neoadjuvant chemotherapy.9
aThe subgroup includes patients who had not received cisplatin-based NAC and were not eligible for or refused
adjuvant cisplatin chemotherapy.
Adapted from Milowsky MI et al.9
DFS: disease-free survival; HR: hazard ratio; MIBC: muscle-invasive bladder cancer; NAC: neoadjuvant chemotherapy; NIVO: nivolumab; No: number; PBO: placebo.
Adjuvant Nivolumab Prolongs Overall Survival
In interim OS analysis for the entire MIBC cohort, the median OS was not reached in the nivolumab group, compared to 39.9 months in the placebo group (HR: 0.70; 95% CI: 0.55–0.90), indicating a 30% reduction in the risk of death with adjuvant nivolumab (Figure 2). In the subgroup of patients with PD-L1 expression ≥1%, the survival difference between the treatment arms was more pronounced, with median OS not reached in the nivolumab group versus 37.6 months in the placebo group (HR: 0.48; 95% CI: 0.29–0.77; Figure 2).
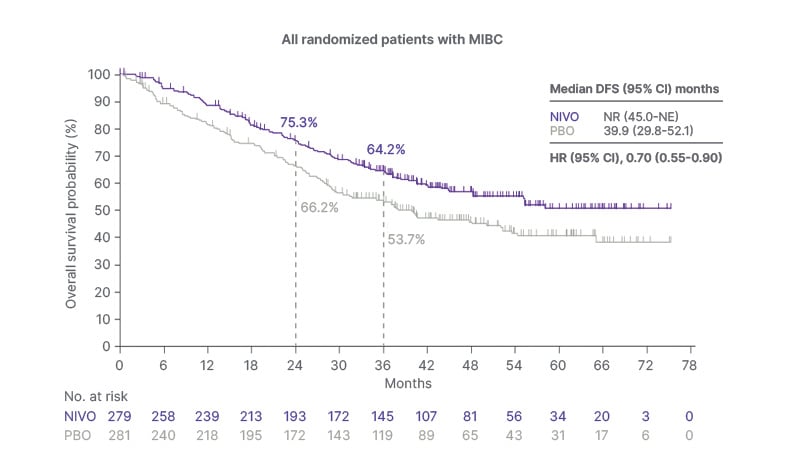
Figure 2: Kaplan-Meier curves showing overall survival in all randomized patients with muscle-invasive bladder cancer.9
aInterim OS analysis.
Median follow-up of 36.1 months in the intent-to-treat population and 34.5 months in the MIBC population.
Adapted from Milowsky MI et al.9
HR: hazard ratio; MIBC: muscle-invasive bladder cancer; NE: not estimable; NR: not reached; NIVO: nivolumab; OS: overall survival; PBO: placebo.
Interim analysis of OS based on treatment history indicated that adjuvant nivolumab provided a survival benefit regardless of prior treatment with NAC. Among patients who received prior NAC, the median OS was 55.2 months with nivolumab, compared to 40.2 months with placebo (HR: 0.74; 95% CI: 0.53–1.03; Figure 2). Among patients without prior NAC, the median OS was not reached in the nivolumab group, versus 37.7 months in the placebo group (HR: 0.67; 95% CI: 0.47–0.95; Figure 2).
No New Safety Signals
The extended follow-up analysis of patients with MIBC treated with adjuvant nivolumab revealed no new safety signals.9,11 The toxicity profile of adjuvant nivolumab in patients with MIBC was consistent with that reported in patients with MIUC.7 Treatment-related adverse events (TRAE) were reported in 80% of nivolumab-treated patients and 56% of placebo-treated patients.9,11 Grade ≥3 TRAEs were reported in 17% of patients in the nivolumab group and 6% of those in the placebo group, and the rate of TRAEs leading to discontinuation was 15% and 2%, respectively.9,11 The most common TRAEs in patients treated with adjuvant nivolumab included pruritus, fatigue, and diarrhea.9,11
Implications
Milowsky noted that the results of this extended follow-up analysis of the MIBC sub-cohort reinforce the role of nivolumab as a standard of care in the adjuvant setting for high-risk MIUC, including MIBC, following radical surgery. The benefit was observed regardless of PD-L1 status or prior NAC, supporting the utility of nivolumab in the broader MIBC population. The favorable OS trends, particularly in patients with PD-L1 ≥1%, indicate potential long-term survival benefits, and the manageable safety profile supports the feasibility of long-term treatment.
Milowsky added that the recent FDA approval of subcutaneous nivolumab for various solid tumors, including urothelial carcinoma,12 offers an additional administration option that may reduce treatment burden. He concluded that the observed efficacy, along with a favorable benefit-risk ratio, potentially provide an opportunity for a curative outcome.