Meeting Summary
Overall survival with rare, highly aggressive, poorly differentiated extrapulmonary neuroendocrine carcinomas (epNEC) is typically short. Platinum-based chemotherapy is the only standard-of-care treatment used first-line, with no standard-of-care for second-line and beyond. The Notch signaling pathway inhibitor delta-like ligand 3 (DLL3) is expressed in around 80% of epNECs. Obrixtamig, a novel DLL3/CD3 IgG-like T cell engager under clinical investigation, is designed to induce T cell redirected lysis of cancer cells expressing DLL3 on the cell surface. At the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, Jaume Capdevila presented data from an ongoing Phase I dose-escalation trial of obrixtamig in patients with DLL3-positive epNEC who have failed standard treatment. This analysis examined the efficacy and safety of obrixtamig in patients with DLL3high (n=30) versus DLL3low (n=30) expression. The trial utilized a step-up dosing strategy for obrixtamig to mitigate immune-mediated toxicities. Obrixtamig demonstrated a manageable safety profile. While cytokine release syndrome occurred in 65%, this was Grade ≥3 in only 3%. Potential neurological adverse events (AE), including immune effector cell-associated neurotoxicity syndrome, occurred in 13% of patients, with 5% at Grade ≥3. This analysis showed an encouraging objective response rate (ORR) of 40% (95% CI: 25−58%) in the DLL3high subgroup, and 3% (95% CI: 1−17%) in the DLL3low subgroup. Disease control rates (DCR) were 67% and 27%, with a median duration of response (mDoR) of 7.9 and 2.8 months, respectively. In conclusion, obrixtamig showed a manageable safety profile in all patients with epNEC in this trial. The ORR of 40% in patients in the DLL3high subgroup is promising in the context of typical response rates with chemotherapy (~0–25%), warranting further investigation. An ongoing Phase II trial (DAREON®-5) is investigating obrixtamig in patients with DLL3high epNEC who progressed on one prior line of platinum-based therapy.
Introduction
EpNECs are considered rare and are often associated with a poor prognosis, with up to 85% of patients presenting with advanced, unresectable disease.1 First-line platinum-based chemotherapy is the only standard-of-care treatment. Disease progression typically occurs within months of receiving first-line chemotherapy, with a median progression-free survival of around 4−9 months, and a median overall survival of around 5−16 months.2-6 Currently, there is no standard second-line chemotherapy, with limited trials of various regimens showing ORRs of ≤25%.7
Around 80% of epNECs express DLL3, a Notch signaling pathway inhibitor involved principally in embryonic development, but also in tumorigenesis.8 Because DLL3 is minimally expressed in normal tissue, it is a target for investigational treatment strategies in epNEC.9
Obrixtamig, an IgG-like bispecific (DLL3/CD3) T cell engager, redirects a patient’s T cells to destroy DLL3 positive cancer cells. It acts by binding both DLL3 and CD3, bringing T cells into close proximity with DLL3-positive cancer cells to form a major histocompatibility complex-independent immune synapse, leading to T cell-induced lysis of the DLL3 positive cancer cells.10,11 Obrixtamig, which is an investigational agent not approved outside of clinical trial use, gained U.S. FDA orphan drug12 and fast track13 designations for epNEC in 2023, with orphan drug designation by the EMA in 2024.14
Efficacy and Safety of Obrixtamig in a Phase I Trial
At ASCO 2025, Capdevila presented an ongoing, Phase I dose-escalation trial of obrixtamig.15 The study includes patients with DLL3-positive epNEC who failed, or were ineligible for, standard treatment, and have adequate liver, bone marrow, and renal function. Intravenous obrixtamig (active dose range 90−1,080 µg/kg) is administered weekly or every 3 weeks after the first three weekly injections until disease progression or unacceptable toxicity. It is administered in a step-up dosing strategy to mitigate immune-mediated toxicities. Primary endpoints are maximum tolerated dose, and dose-limiting toxicities. Secondary endpoints include ORR, and pharmacokinetic parameters.15,16
Of the 60 patients with epNEC presented here, 30 were ‘DLL3high‘ (≥50% tumor cells staining at moderate and/or strong intensity) and 30 were ‘DLL3low‘ (<50% tumor cells staining at moderate and/or strong intensity). Median age was similar between the subgroups (DLL3high: 69 [range 36−81] years; DLL3low: 61 [range 33−77] years); 57% in the DLL3high subgroup and 87% in the DLL3low subgroup were male. Primary origin classification was gastrointestinal (47% DLL3high; 60% DLL3low), genitourinary (40% DLL3high; 27% DLL3low), and cancer of unknown primary site (13% DLL3high; 10% DLL3low). Eastern Cooperative Oncology Group performance status was balanced between the DLL3 subgroups. Approximately 67% of patients in the DLL3high subgroup and 77% in the DLL3low subgroup had received ≥2 prior lines of therapy,16 underscoring the poor prognosis of this population.
Safety
Overall, 95% of patients experienced a treatment-related AE, with 22% experiencing Grade ≥3 AEs. While 65% experienced cytokine release syndrome of any Grade, this was Grade ≥3 in only 3%. Other reported AEs included pyrexia (32%), dysgeusia (25%), fatigue (18%), and decreased appetite (17%), none of which were Grade ≥3. Asthenia (23%) and decreased lymphocyte count (15%) were Grade ≥3 in 2% and 12% of patients, respectively. Potential neurological AEs, including immune effector cell-associated neurotoxicity syndrome, occurred in 13% overall, with 5% being Grade ≥3.16
Efficacy
At data cutoff (June 21st 2024), in the DLL3high subgroup, ORR was 40% (95% CI: 25−58%), and DCR was 67% (95% CI: 49−81%), with stable disease in 27%. In the DLL3low subgroup, ORR was 3% (95% CI: 1−17%), and DCR was 27% (95% CI: 14−44%), with stable disease in 23%. Progressive disease occurred in 27% of evaluable patients in the DLL3high subgroup (7% not-evaluable [NE]) and in 50% of the evaluable patients in the DLL3low subgroup (23% NE). Similar efficacy was observed regardless of tumor origin.16
As illustrated in Figures 1 and 2, a higher proportion of patients in the DLL3high subgroup achieved partial responses (PR), or tumor shrinkage, compared with those in the DLL3low subgroup.16
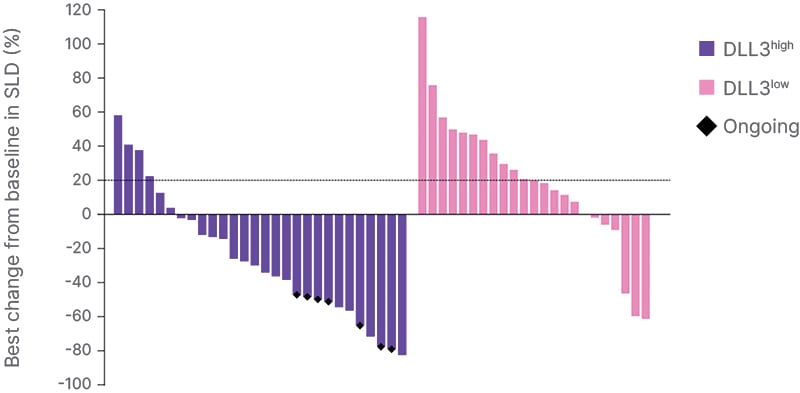
Figure 1: Best change from baseline in sum of lesion diameter.
Data cutoff June 21st 2024.
DLL3: delta-like ligand 3; SLD: sum of lesion diameter.
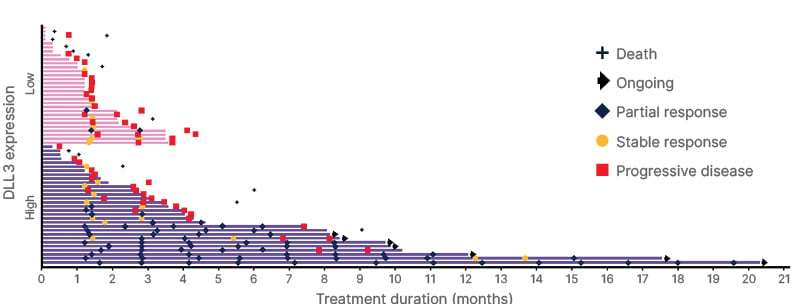
Figure 2: Patient duration of response by DLL3 expression.
Data cutoff June 21st 2024.
DLL3: delta-like ligand 3.
At a median follow-up of 9.7 months (95% CI: 6.5−13.9), while mDoR in the DLL3high subgroup was 7.9 months (95% CI: 6.2−NE), with seven patients remaining on treatment at data cutoff, in the DLL3low subgroup (95% CI: NE−NE), mDoR was 2.8 months. PR was observed within the first 1–3 months in approximately half of the DLL3highsubgroup (Figure 2).16
Case Study
Figure 3 illustrates the case of a 79-year-old female diagnosed in September 2021 with multiple liver epNEC lesions. Obrixtamig was initiated following the discontinuation of first-line chemotherapy. The patient achieved a PR after 6 weeks of obrixtamig, and has continued obrixtamig treatment, maintaining good tolerability 2.5 years later.16
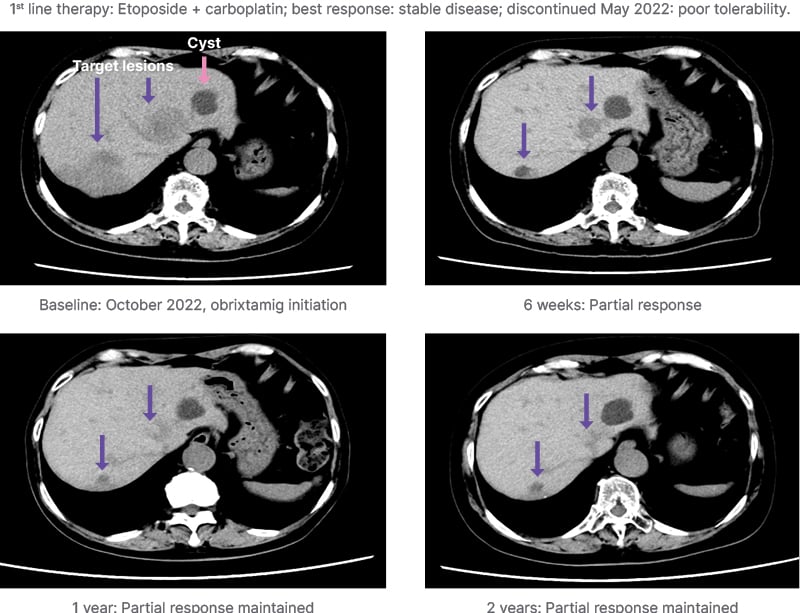
Figure 3: Case study: DLL3high extrapulmonary neuroendocrine carcinoma.
Provided by Yasutoshi Kuboki, Department of Experimental Therapeutics, National Cancer Center Hospital East, Kashiwa, Japan. Non-contrast CT images, due to patient’s allergy to contrast agent.
Conclusion
To date, this is the largest prospective dataset for a T cell engager in patients with epNEC. Obrixtamig demonstrated a manageable safety profile, with an ORR of 40% and durable responses observed in the DLL3high subgroup.16 An ongoing Phase II trial (DAREON®-5) is investigating obrixtamig in patients with relapsed or refractory DLL3high epNEC.17 DAREON®-7 is an ongoing Phase I trial of obrixtamig in combination with platinum and etoposide in patients with DLL3-positive tumors.18







