Author: Christos Evangelou1
1. MedRight Medical Writing & Editing, San Diego, California, USA
Disclosure: Evangelou has declared no conflicts of interest.
Support: The publication of this article was funded by AstraZeneca.
Keywords: Antibody–drug conjugates (ADC), circulating tumor DNA (ctDNA), endo¬crine therapy, ESR1 mutations, hormone receptor-positive (HR+) advanced breast cancer, selective estrogen receptor degrader (SERD), targeted therapy.
Citation: Oncol AMJ. 2025;2[1]:40-51. https://doi.org/10.33590/oncolamj/CTRC4560
Summary
The therapeutic landscape for hormone receptor-positive (HR+) advanced breast cancer (BC) is moving from generalized endocrine therapies to highly targeted, biomarker-driven strategies. This report synthesizes recent advancements in the treatment of HR+ advanced BC presented at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting (May 30th–June 3rd, 2025, Chicago, Illinois, USA), highlighting the role of biomarker-guided interventions. A key development is the ability to identify resistance mechanisms early, particularly ESR1 mutations via circulating tumor DNA (ctDNA), which can inform treatment decisions. Next-generation endocrine therapies, including novel oral selective estrogen receptor degraders (SERD) and proteolysis-targeting chimeras (PROTAC), along with targeted combinations such as PI3K inhibitors, are demonstrating improvements in progression-free survival (PFS) in specific patient populations. Furthermore, antibody–drug conjugates (ADC) offer new treatment options beyond first line with enhanced efficacy compared to chemotherapy. The overarching treatment trajectory for HR+ advanced BC points toward a molecular surveillance paradigm, where precision diagnostics inform proactive and individualized treatment sequences, aiming to extend the duration of therapeutic benefit and improve patient quality of life (QoL).
INTRODUCTION
HR+ BC is the most common subtype of advanced BC, representing approximately 65–77% of all advanced cases.1-3 HR+ BC is characterized by the expression of estrogen receptor (ER), progesterone receptor (PR), or both, which are targets of endocrine therapy and targeted therapies.4 Most HR+ BCs lack expression of human epidermal growth factor receptor 2 (HER2).3
The introduction of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors over a decade ago has improved PFS and overall survival (OS) for patients with advanced HR+ BC.5-7 The combination of endocrine therapy with CDK4/6 inhibitors is the current standard of care (SOC) for most previously untreated patients with advanced HR+ BC, providing a median OS of 24–60 months and PFS of 6–60 months.5,8
For patients who progress on first-line CDK4/6 inhibitor-based therapy, treatment options include SERDs administered intramuscularly, such as fulvestrant, or the oral SERD elacestrant.9 Elacestrant was approved by the FDA in 2023 based on results from the Phase III EMERALD trial and was the first oral SERD to demonstrate clinical benefit in patients with ER+/HER2- advanced BC who had received prior CDK4/6 inhibitor therapy.9-11 In the EMERALD trial, elacestrant significantly prolonged PFS compared to standard endocrine therapy (fulvestrant or an aromatase inhibitor), particularly in patients with ESR1-mutant tumors (median PFS: 3.8 versus 1.9 months; hazard ratio [HR]: 0.55; p=0.0005). 11
For patients with HR+ advanced BC harboring specific molecular alterations, targeted therapies are available. The PI3K/AKT/mTOR pathway, frequently activated in HR+ BC, can be targeted with agents such as alpelisib (approved for use in combination with fulvestrant in patients with PIK3CA-mutated tumors), inavolisib (approved for use in combination with palbociclib and fulvestrant in patients with PIK3CA-mutated tumors), and capivasertib (approved for patients with AKT pathway alterations).9 The mTOR inhibitor everolimus, in combination with endocrine therapy, is another option for patients with advanced HR+ BC that has progressed after prior endocrine therapy.12
However, most patients with advanced HR+ BC eventually progress, as resistance to endocrine therapy emerges.7,13 Common mechanisms of treatment resistance in HR+ BC include the emergence of mutations in the estrogen receptor 1 gene (ESR1), which can lead to estrogen-independent growth of HR+ BC; loss of ER or PR expression, which makes cancer cells independent of estrogen stimulation and resistant to endocrine therapies; and activation of upstream growth factor signaling pathways, such as the PI3K/Akt/mTOR pathway, which can bypass the need for estrogen signaling.14,15 Novel therapeutic strategies that can overcome resistance mechanisms are needed to extend the duration of disease control and improve QoL in patients with advanced HR+ BC.16
The most recent developments in advanced HR+ BC include novel biomarker-guided strategies, next-generation endocrine therapies, targeted combinations, and ADCs.17 This review provides an up-to-date synthesis of the latest clinical data from recent Phase III trials in advanced HR+ BC, including data on novel ctDNA-guided switch strategies, next-generation endocrine therapies, targeted combinations, and ADCs, presented at the 2025 ASCO Annual Meeting (Table 1). This review of recent clinical data aims to support informed clinical decision making among clinicians treating patients with advanced HR+ BC.
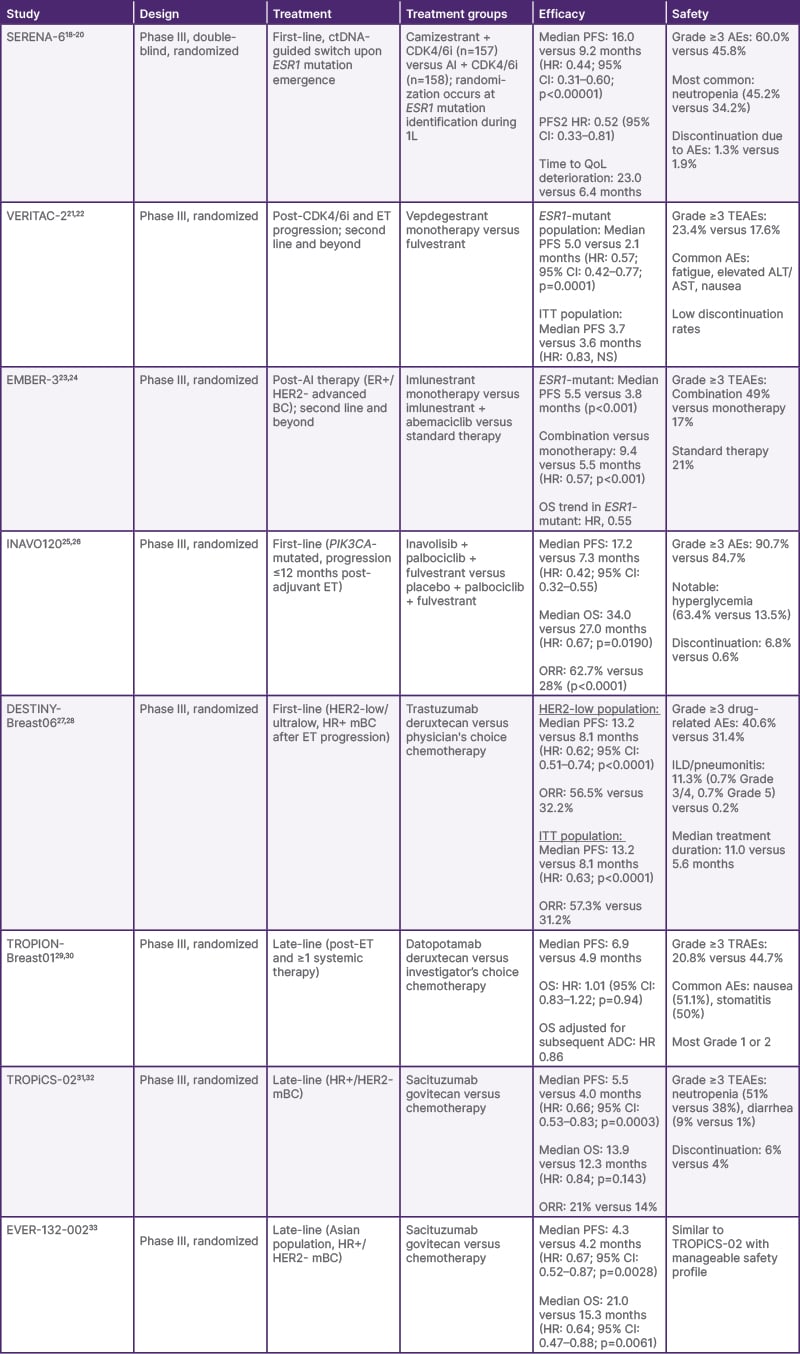
Table 1: Summary of latest data from recent Phase III trials in advanced hormone receptor-positive breast cancer.
ADC: antibody–drug conjugate; AE: adverse event; AI: aromatase inhibitor; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BC: breast cancer; CDK4/6i: cyclin-dependent kinase 4/6 inhibitor; CI: confidence interval; ctDNA: circulating tumor DNA; ER: estrogen receptor; ET: endocrine therapy; HER2: human epidermal growth factor receptor 2; HR: hazard ratio; HR+: hormone receptor-positive; ITT: intent-to-treat; mBC: metastatic breast cancer; NS: not significant; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; PFS2: time to second disease progression; QoL: quality of life; TEAE: treatment-emergent adverse event; TRAE: treatment-related adverse event; 1L: first-line.
ADVANCES IN ctDNA-GUIDED TREATMENT SWITCHING: INSIGHTS FROM SERENA-6
Analysis of ctDNA levels is increasingly used in oncology, offering a non-invasive method for monitoring tumor evolution and treatment response.34,35 In advanced HR+ BC, ctDNA has been used to detect the emergence of resistance mutations, such as those in the ESR1 gene, which are frequently associated with acquired resistance to endocrine therapy and disease progression.36-38 In early proof-of-concept studies in advanced lung cancer and hepatocellular carcinoma, analysis of ctDNA enabled the identification of resistance mutations before clinical or radiographic progression, or when tissue biopsies were unavailable.39,40 As such, analysis of ctDNA levels provides a proactive, minimally invasive approach to clinical decision making based on molecular signals rather than waiting for measurable, macroscopic disease progression.35 Figure 1 illustrates key differences between the current ESR1 testing approach and the investigational testing strategy being evaluated in clinical trials such as SERENA-6 and PADA-1.18-20,41
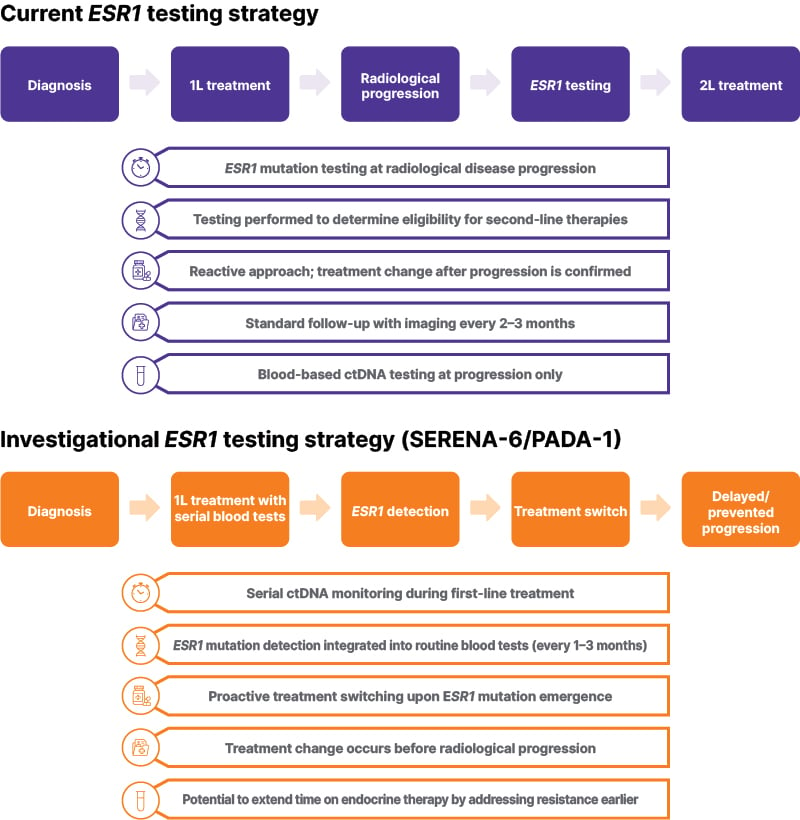
Figure 1: Comparison of current and investigational ESR1 testing strategies in HR+/HER2- advanced breast cancer.
The current standard approach tests for ESR1 mutations at radiological disease progression to guide second-line therapy selection. The investigational strategy, evaluated in trials such as SERENA-6 and PADA-1, incorporates serial ctDNA monitoring to detect emerging ESR1 mutations before progression.
ctDNA: circulating tumor DNA; 1L: first-line; 2L: second-line.
The ongoing SERENA-6 study is the first global, double-blind, registrational Phase III trial to implement a ctDNA-guided approach for early intervention in advanced HR+ BC.18,19 The study is evaluating the efficacy and safety of switching to camizestrant, a next-generation oral SERD, in combination with a CDK4/6 inhibitor, upon detection of an emergent ESR1 mutation in patients receiving first-line aromatase inhibitor plus CDK4/6 inhibitor therapy, ahead of disease progression.18-20
Among the 3,256 participants screened for ESR1 mutations using ctDNA, 548 patients with HR+/HER2- advanced BC were identified with an emergent ESR1 mutation while on first-line aromatase inhibitor plus CDK4/6 inhibitor treatment, and 315 were randomized into step 2 of the study.19,20 The identification of ESR1 mutations in these 548 patients was only during the surveillance step before they closed screening, when 315 were recruited for randomization. Patients were randomized to either switch to camizestrant in combination with their existing CDK4/6 inhibitor (palbociclib, ribociclib, or abemaciclib; n=157) or continue with their SOC aromatase inhibitor plus CDK4/6 inhibitor (n=158).19,20
An interim analysis of SERENA-6 presented at ASCO 2025 demonstrated a statistically significant improvement in PFS, the primary study endpoint, with camizestrant compared with SOC.19,20 At a median follow-up of 12.6 months, the median PFS was 16.0 months in the camizestrant combination arm, compared to 9.2 months in the aromatase inhibitor combination arm, representing a 56% reduction in the risk of disease progression or death (HR: 0.44; 95% CI: 0.31–0.60; p<0.00001) for patients switching to camizestrant on identification of emerging resistance.19,20 This PFS benefit was observed across all CDK4/6 inhibitors and various clinically relevant subgroups, including age, race, region, and type of ESR1 mutation.19,20 Data for OS were immature at the time of this interim analysis (only 12% maturity), but a trend toward extended treatment benefit in the camizestrant arm was observed with time to second disease progression (PFS2; defined as the time from randomization to progression on the next line of therapy or death from any cause), with an HR of 0.52 (95% CI: 0.33–0.81; 27% maturity).19
In addition to the PFS benefit, the switch to camizestrant was also associated with a significant delay in the deterioration of patient-reported QoL. The study showed a 47% reduced risk of deterioration in global health status and QoL, as measured on the 30-item European Organization for the Research and Treatment of Cancer (EORTC) QoL questionnaire, compared with continued aromatase inhibitor treatment.20 The median time to deterioration of global health status and QoL was 23.0 months in the camizestrant arm versus 6.4 months in the aromatase inhibitor arm (HR: 0.53; 95% CI: 0.33–0.82).20
The safety profile of camizestrant in combination with CDK4/6 inhibitors was consistent with the known safety profiles of the individual agents. 20 Although Grade 3 or higher adverse events (AE) were more frequent in the camizestrant arm (60.0% versus 45.8%), these AEs were predominantly hematologic events typically associated with CDK4/6 inhibitor treatment.20 The most frequent AE of Grade 3 or higher was neutropenia (45.2% in the camizestrant group versus 34.2% in the aromatase inhibitor group). 20 Discontinuation rates due to AEs were very low and similar between the two arms (1.3% for camizestrant, 1.9% for the aromatase inhibitor).20
SERENA-6 is the first Phase III trial to demonstrate the clinical utility of early, ctDNA-driven intervention in advanced HR+ BC. 19 It introduces a novel molecular surveillance paradigm where the detection of emergent ESR1 mutations before disease progression can inform a proactive and early treatment switch to potentially extend the benefit of endocrine therapy during first-line treatment.19 In addition, camizestrant is the first next-generation oral SERD and complete ER antagonist to demonstrate consistent PFS benefit in combination with widely approved CDK4/6 inhibitors in this first-line setting.19,20 The results of SERENA-6 suggest that camizestrant could be a promising new standard endocrine therapy backbone for HR+ BC.42 Based on the results of the Phase III SERENA-6 trial, the FDA has granted Breakthrough Therapy Designation for camizestrant in combination with a CDK4/6 inhibitor (palbociclib, ribociclib, or abemaciclib) for the treatment of HR+/HER2- locally advanced or metastatic BC upon emergence of ESR1 mutation during first-line endocrine therapy.42 An ongoing Phase III trial, SERENA-4, is investigating the efficacy and safety of camizestrant plus palbociclib, versus anastrozole plus palbociclib, in patients with ER+/HER2- advanced BC who have not previously received systemic treatment for advanced disease.43
RECENT ADVANCES IN NEXT-GENERATION ENDOCRINE STRATEGIES
The landscape of endocrine therapy for HR+ advanced BC has evolved in recent years, with the development of novel agents that aim to overcome resistance mechanisms, particularly ESR1 mutations.14 Next-generation endocrine therapies for HR+ advanced BC include SERDs and PROTACs.14
EMBER-3: A Phase III Study of Imlunestrant in Post-CDK4/6 Setting
The Phase III EMBER-3 trial explored the efficacy of imlunestrant, an oral SERD, both as monotherapy and in combination with the CDK4/6 inhibitor abemaciclib, in patients with ER+/HER2- advanced BC that recurred or progressed during or after aromatase inhibitor therapy.23 In the ESR1-mutant population (n=256), imlunestrant monotherapy provided a median PFS of 5.5 months, compared with 3.8 months with standard therapy (p<0.001).23 Although imlunestrant monotherapy did not show a statistically significant PFS benefit over standard therapy in the overall population (median PFS: 5.6 months versus 5.5 months; HR: 0.87; p=0.12), the combination of imlunestrant and abemaciclib demonstrated a significant improvement in PFS in all comers (median PFS: 9.4 months with imlunestrant plus abemaciclib versus 5.5 months with imlunestrant alone; HR: 0.57; p<0.001).23 Interim analysis suggests a favorable OS trend for imlunestrant in patients who are ESR1-mutant (HR: 0.55; 95% CI: 0.35–0.86), but did not meet formal significance thresholds.23
Results of a subgroup analysis of patient-reported outcomes among patients with ESR1 mutations were presented at the 2025 ASCO Annual Meeting. The analysis showed that imlunestrant improved or maintained global health status, QoL, and physical function.44 The 2025 ASCO Annual Meeting also featured data from a safety analysis in the EMBER-3 cohort, showing higher rates of Grade 3 or higher treatment-emergent adverse events (TEAE) with the combination arm (49%) compared with monotherapy arms (21% for standard therapy, 17% for imlunestrant monotherapy). 24
Early Clinical Data on Giredestrant
Giredestrant is another oral SERD under investigation. The Phase II acelERA trial compared giredestrant to physician’s choice of endocrine monotherapy (fulvestrant or aromatase inhibitors) in patients with ER+/HER2- advanced BC who had progressed after one or two prior systemic therapies.45 Although the primary endpoint of investigator-assessed PFS was not met (HR: 0.81; 95% CI: 0.60–1.10; p=0.1757), a trend toward favorable benefit was observed in the ESR1-mutant subgroup (HR: 0.60; 95% CI: 0.35–1.03). 45 Giredestrant was well tolerated, with a safety profile consistent with that of standard endocrine therapies.45 Although it did not demonstrate a statistically significant PFS benefit, giredestrant showed a manageable safety profile and efficacy signals in patients with ESR1-mutated tumors, supporting further investigation in larger trials.45
The ongoing randomized, double-blind, Phase III persevERA trial is evaluating giredestrant in combination with palbociclib versus letrozole plus palbociclib in the first-line setting for ER+/HER2- advanced or metastatic BC.46 The primary endpoint is PFS per RECIST v1.1, and key secondary endpoints include OS, objective response rate (ORR), duration of response, QoL, and safety.46 Global enrollment began in October 2020, and the expected primary completion is in late 2025.46
VERITAC-2: A Phase III Study of a Proteolysis-Targeting Chimeras Estrogen Receptor Degrader
The Phase III VERITAC-2 trial investigated vepdegestrant, an investigational oral PROTAC ER degrader, as monotherapy against fulvestrant in patients with ER+/HER2- advanced or metastatic BC whose disease progressed after treatment with CDK4/6 inhibitors and endocrine therapy.21 VERITAC-2 met its primary endpoint, demonstrating a statistically significant improvement in PFS within the ESR1-mutant population, surpassing the pre-specified target HR of 0.60 in this subgroup.21,22 According to data presented at the 2025 ASCO Annual Meeting, the median PFS was 5.0 months in the vepdegestrant arm and 2.1 months in the fulvestrant arm (HR: 0.57; 95% CI: 0.42–0.77; p=0.0001).22 However, the trial did not reach statistical significance for PFS in the intent-to-treat (ITT) population (median PFS: 3.7 months versus 3.6 months; HR: 0.83; 95% CI: 0.68–1.02; p=0.0358).22 OS data were immature at the time of analysis. Vepdegestrant was generally well tolerated, with low rates of discontinuation due to TEAEs in both arms.22 Grade ≥3 TEAEs occurred in 23.4% of patients in the vepdegestrant group and 17.6% in the fulvestrant group.22 Common AEs with vepdegestrant included fatigue, elevated liver transaminases, and nausea.22
VERITAC-2 was the first head-to-head study to compare the efficacy and safety of vepdegestrant with the intramuscular SERD fulvestrant in patients with advanced ER+/HER2- BC after combination CDK4/6 inhibitor therapy and endocrine therapy.21 The results of VERITAC-2 support vepdegestrant as a potential treatment option for previously treated patients with advanced ER+/HER2- BC with ESR1 mutations.22 The findings also underscore the importance of ESR1 mutation testing in guiding treatment decisions for patients who have progressed on prior CDK4/6 inhibitor-based regimens.21,22
TARGETED COMBINATIONS
Combination therapies targeting the PI3K/AKT/mTOR pathway have been investigated for the treatment of patients with HR+ advanced BC harboring PIK3CA mutations. The Phase III INAVO120 trial investigated the combination of the PI3K inhibitor inavolisib with the CDK4/6 inhibitor palbociclib and ER degrader fulvestrant versus placebo plus palbociclib and fulvestrant in patients with PIK3CA-mutated, HR+/HER2- locally advanced or metastatic BC.25 Patients included in the study had either progressed during or within 12 months of completing adjuvant endocrine therapy, and had not received prior systemic therapy for metastatic disease.25 The primary analysis showed significant improvement in PFS with inavolisib plus palbociclib–fulvestrant compared with placebo palbociclib–fulvestrant, leading to FDA approval of inavolisib in combination with palbociclib and fulvestrant for the treatment of PIK3CA-mutated, HR+/HER2- locally advanced or metastatic BC following recurrence on or after completing adjuvant endocrine therapy.25,47
Final analysis of INAVO120 was presented at the 2025 ASCO Annual Meeting, and showed that, with a median follow-up of 34.2 months, the median OS was 34.0 months for patients in the inavolisib group, versus 27.0 months for those in the placebo group, representing a 33% reduction in the risk of death with the inavolisib combination (stratified HR: 0.67; 95% CI: 0.48–0.94; p=0.0190).26 The median PFS was 17.2 months in the inavolisib triplet arm, compared to 7.3 months in the placebo arm (HR: 0.42; 95% CI: 0.32–0.55).26 The inavolisib regimen also significantly delayed the time until patients required chemotherapy, with a median of 35.6 months in the inavolisib group, versus 12.6 months in the placebo group (stratified HR: 0.43; 95% CI: 0.30–0.60).26 The ORR was also higher in the inavolisib arm (62.7% versus 28%; p<0.0001).26
Grade 3 or higher AEs were more common in the inavolisib group (90.7% versus 84.7%), with hyperglycemia being a common side effect (63.4% versus 13.5%).26 However, discontinuation rates due to side effects were low (6.8% versus 0.6%).26
ADVANCES IN ANTIBODY–DRUG CONJUGATES
ADCs have been explored as treatment options for patients with HR+ advanced BC.48 ADCs combine the specificity of monoclonal antibodies with the cytotoxic activity of chemotherapy, delivering the payload directly to cancer cells expressing specific targets.49 ADCs tested in patients with HR+ advanced BC include trastuzumab deruxtecan (T-DXd), datopotamab deruxtecan (Dato-DXd), and sacituzumab govitecan.48
Trastuzumab Deruxtecan
The Phase III DESTINY-Breast04 trial established T-DXd as a SOC for patients with HER2-low metastatic BC who had received prior chemotherapy, improving PFS and OS compared with physician’s choice of chemotherapy.50 The Phase III DESTINY-Breast06 trial evaluated the efficacy of T-DXd in the earlier treatment setting for patients with HER2-low, HR+ metastatic BC who had received no more than one prior line of chemotherapy.27 The study met its primary endpoint, demonstrating a statistically significant improvement in PFS with T-DXd compared with investigator’s choice of chemotherapy (median PFS: 13.2 versus 8.1 months; HR: 0.62; p<0.0001).27 Exploratory biomarker analysis presented at the 2025 ASCO Annual Meeting showed that in all biomarker-defined subgroups (PI3K pathway mutations, ESR1 mutations, BRCA1/2 mutations), T-DXd outperformed physician’s choice chemotherapy for both PFS and confirmed ORR.28 The safety profile of T-DXd was consistent with previous studies, with drug-related interstitial lung disease or pneumonitis occurring in approximately 10–15% of patients, though most cases were low-grade and manageable.27,50
Datopotamab Deruxtecan
The Phase III TROPION Breast01 trial evaluated the efficacy and safety of Dato-DXd against investigator’s choice of chemotherapy in patients with unresectable or metastatic HR+/HER2- BC who had progressed on endocrine-based therapy and at least one additional systemic therapy.51
The trial met its dual primary endpoint of PFS, demonstrating a significant and clinically meaningful improvement with Dato-DXd compared with chemotherapy (median PFS: 6.9 months versus 4.9 months; HR: 0.63; 95% CI: 0.52–0.76; p<0.0001).29 However, the study did not meet the second primary endpoint of OS, according to final OS analysis data presented at the 2025 European Society for Medical Oncology (ESMO) Virtual Plenary (HR: 1.01; 95% CI: 0.83–1.22; p=0.94).30 Imbalances between the two groups in the use of other ADCs (T-DXd and sacituzumab govitecan) as subsequent therapy (12.3% in Dato-DXd arm versus 24.0% in the chemotherapy arm) may have confounded the impact of Dato-DXd on OS in this population, as OS sensitivity analysis for subsequent ADC use showed an adjusted HR of 0.86 (95% CI: 0.70‒1.06).30
The safety profile of Dato-DXd was consistent with previous observations, showing lower rates of Grade 3 or higher treatment-related adverse events (TRAE) compared to chemotherapy (20.8% versus 44.7%).29 The most common any-grade TRAEs with Dato-DXd were nausea (51.1%) and stomatitis (50.0%), with most cases being Grade 1 or 2. 29 These findings suggest that Dato-DXd provides a new post-chemotherapy option for patients with HR+ advanced BC, especially for those with HER2-negative tumors, offering improved tolerability compared with standard chemotherapy.29 Dato-DXd received FDA approval in January 2025 for this indication.52
Sacituzumab Govitecan
The Phase III TROPiCS-02 study compared sacituzumab govitecan with chemotherapy in patients with HR+/HER2- metastatic BC.31,32 The trial demonstrated a statistically significant improvement in PFS (primary endpoint), with a median PFS of 5.5 months for sacituzumab govitecan versus 4.0 months for chemotherapy (HR: 0.66; 95% CI: 0.53–0.83; p=0.0003).31 Furthermore, sacituzumab govitecan showed a numeric but not statistically significant improvement in OS, with a median OS of 13.9 months versus 12.3 months (HR: 0.84; p=0.143).32 The ORR was 21% for sacituzumab govitecan versus 14% for chemotherapy.32
Another Phase III study, EVER-132-002, evaluated sacituzumab govitecan in Asian patients with HR+/HER2- metastatic BC; the study met its primary endpoint of PFS.33 In this population, sacituzumab govitecan significantly improved PFS (median PFS: 4.3 months versus 4.2 months; HR: 0.67; 95% CI: 0.52–0.87; p=0.0028) and OS (median OS: 21.0 months versus 15.3 months; HR: 0.64; 95% CI: 0.47–0.88; p=0.0061) compared with chemotherapy.33
The safety profile of sacituzumab govitecan was manageable.31 The most common Grade ≥3 TEAEs were neutropenia (51% versus 38%) and diarrhea (9% versus 1%).31 Similar rates of treatment discontinuation due to AEs were reported (6% versus 4%).31
Sacituzumab govitecan has been approved for the treatment of HR+ metastatic BC after endocrine-based therapy and at least two additional systemic therapies, reinforcing its role as an effective treatment option and expanding the therapeutic landscape for patients who have exhausted endocrine-based therapies.53
CONCLUSION
The recent Phase III clinical trials discussed in this article demonstrated that the treatment landscape for advanced HR+ BC is moving from generalized endocrine therapies to biomarker-driven treatment strategies and novel therapeutic mechanisms.17
SERENA-6 has established a ctDNA-guided approach that enables earlier treatment switching for patients with emerging ESR1-mediated resistance.19,20,42 This proactive switch in therapy based on molecular signals in ctDNA can help optimize clinical decision making and improve treatment outcomes for patients with HR+/HER2- advanced BC who have emergent ESR1 mutations.19,20,42
Recent advances in ESR1-targeted therapies, including next-generation SERDs (e.g., camizestrant and elacestrant) and PROTACs (e.g., vepdegestrant), reinforce the importance of molecular testing in the post-CDK4/6 inhibitor setting.11,19-22,42 These agents provide effective treatment options that can extend the benefit of endocrine therapy.11,19-22,42
INAVO120 data demonstrate that rational combination strategies can provide clinical benefit in patient populations selected based on molecular testing.26,47 Inavolisib, palbociclib, and fulvestrant provide new treatment options for patients with PIK3CA-mutant tumors, demonstrating significant improvements in both PFS and OS.26,47
In addition, the approval of ADCs such as Dato-DXd and sacituzumab govitecan has expanded therapeutic options beyond first line, particularly for patients who have progressed after endocrine therapy and chemotherapy.29-33,52 These ADCs offer effective and often better-tolerated alternatives for patients with limited treatment options, especially those who are HER2-negative.29-33,52,53