Meeting Summary
Non-small cell lung cancer (NSCLC) harboring mutations in human epidermal growth factor receptor 2 (HER2) represents a challenging lung cancer subtype with limited treatment options and poor outcomes. Trastuzumab deruxtecan, the only HER2-directed antibody-drug conjugate (ADC) approved for the treatment of HER2-mutant NSCLC, is associated with significant toxicities, including interstitial lung disease. In addition, tyrosine kinase inhibitors (TKI) that are not HER2-specific cause EGFR-related toxicities such as diarrhea and rash. The 2025 American Association for Cancer Research (AACR) Annual Meeting and the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting featured updates on zongertinib, an oral HER2-selective TKI currently under priority review by the FDA for previously treated patients with HER2-mutant advanced NSCLC.
These updates included an oral presentation at AACR that covered efficacy and safety data from the Beamion LUNG-1 Phase Ib trial in three patient cohorts: patients previously treated with double-platinum chemotherapy with or without immunotherapy and HER2 mutations in the tyrosine kinase domain (TKD); patients previously treated with both chemotherapy with or without immunotherapy and, subsequently, HER2-directed ADCs; and patients previously treated with chemotherapy with or without immunotherapy and with HER2 non-TKD mutations. At ASCO, a poster focused on patient-reported outcomes (PRO) from previously treated patients with TKD mutations, examining physical functioning, disease-related symptoms, and treatment tolerability. Together, these presentations demonstrated that zongertinib provides durable clinical activity with a manageable safety profile, and that patients experienced meaningful improvements in symptoms and physical functioning. The data support zongertinib as a treatment option that addresses efficacy, safety, and patient-centered outcomes in previously treated patients with HER2-mutant advanced NSCLC, a patient population that currently has limited therapeutic options.
Introduction
Mutations in HER2, also known as ERBB2, represent a distinct molecular subset of NSCLC, occurring in approximately 2–4% of cases, and are associated with poor prognosis and higher incidence of brain metastases.1 The majority of HER2 mutations in patients with NSCLC occur within the TKD, particularly exon 20 insertions, although non-TKD mutations in the extracellular and transmembrane domains also contribute to disease pathogenesis.2,3 Currently, trastuzumab deruxtecan, a HER2-directed ADC, is the only FDA-approved therapy for previously treated patients with HER2-mutant advanced or metastatic NSCLC, having received accelerated approval in 2022 based on the DESTINY-Lung studies.4,5 However, there remains a need for a TKI that selectively inhibits HER2 while sparing wild-type EGFR, said Heymach.
He explained that targeting HER2 mutations has, to date, been challenging. Reasons include structural complexities that affect drug binding, as well as the difficulty in developing selective inhibitors that spare wild-type EGFR, and thereby avoid associated toxicities such as severe diarrhea and rash.6 Zongertinib (BI 1810631) is an orally administered, irreversible, and potent TKI that demonstrates high selectivity for HER2 and spares wild-type EGFR, limiting EGFR-related toxicities.6,7
Efficacy and Safety of Zongertinib Across Multiple Cohorts
Study Design and Patient Population
The Beamion LUNG-1 Phase Ib dose-expansion study evaluated zongertinib (120 mg once daily) in three cohorts of previously treated patients. The data presented at AACR 2025 represented the first mature time-to-event analysis with a data cutoff of November 29th, 2024.8 Cohort 1 included 75 patients with TKD mutations previously treated with chemotherapy with or without immunotherapy, Cohort 5 comprised 31 patients with TKD mutations previously treated with chemotherapy with or without immunotherapy and, subsequently, HER2-directed ADCs, and Cohort 3 included 20 previously treated patients with non-TKD mutations. Two additional cohorts (Cohort 2 comprising treatment-naive patients with TKD mutations, and Cohort 4 comprising patients with TKD mutations and active brain metastases) were also included in the study, but data for these patients are not yet available.8
Brain metastasis is common in patients with HER2-mutant NSCLC, which was reflected in the substantial proportions of patients with brain metastases at baseline (37% in Cohort 1; 74% in Cohort 5; 40% in Cohort 3).8 The proportion of patients that had received two or more prior treatment lines was also high, ranging from 39% of patients in Cohort 1, to 84% in Cohort 5, and 60% in Cohort 3.8
Durable Clinical Activity of Zongertinib in Previously Treated Patients with TKD Mutations
In Cohort 1 (previously treated patients with TKD mutations), zongertinib demonstrated a confirmed objective response rate (ORR) of 71% (95% CI: 60–80%; p<0.001), including 7% complete responses and 64% partial responses.8 The disease control rate was 96% (95% CI: 89–99%).8 Notably, responses with zongertinib were observed across mutation subtypes, including 81% ORR in patients with A775_G776insYVMA insertions and 75% in those with P780_Y781insGSP insertions.8
Responses were durable, with a median duration of response of 14.1 months (95% CI: 6.9 to not evaluable) and median progression-free survival of 12.4 months (95% CI: 8.2 to not evaluable).8 Responses were rapid, with a median time to objective response of 1.4 months (range: 1.1–6.9 months), and the majority were observed at the first assessment (data presented ahead of publication; Figure 1).
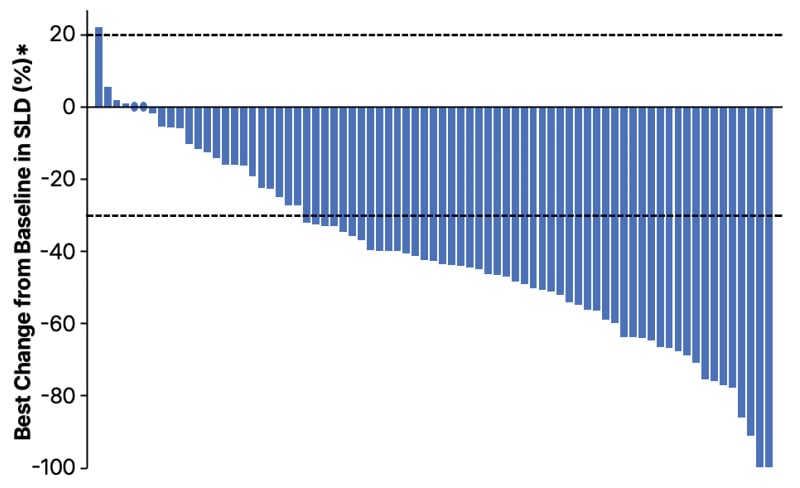
Figure 1: Best percentage change from baseline in target lesion diameter in patients with HER2 tyrosine kinase domain mutations treated with zongertinib 120 mg once daily (N=75).
Each bar represents an individual patient. The objective response rate was 71% (95% CI: 60–80%), with 7% complete responses and 64% partial responses. Disease control rate was 96% (95% CI: 89–99%). Median follow-up was 11.3 months.
*Due to the central review process, lesion measurements are only available for investigator assessment.
SLD: sum of lesion diameters.
Activity in Previously Treated Patients with TKD and Non-TKD Mutations
Zongertinib demonstrated clinical activity in patients who were previously treated with chemotherapy with or without immunotherapy, and subsequently received HER2-directed ADCs (Cohort 5), with an ORR of 48% (95% CI: 32–65%).8 Among the 22 patients who had previously received trastuzumab deruxtecan, the ORR was 41% (95% CI: 23–61%).8 These findings suggest that patients who progressed on previous standard of care therapy may respond to zongertinib, and that resistance mechanisms to trastuzumab deruxtecan may not confer cross-resistance to zongertinib.8
In the exploratory Cohort 3 (n=20; 17 with known activating mutations), previously treated patients with non-TKD HER2 mutations demonstrated an ORR of 30% (95% CI: 15–52%)8 and a disease control rate of 65% (95% CI: 43–82%; data presented ahead of publication). This represents the largest dataset presented for this patient population, with responses observed across different mutation types in the extracellular and transmembrane domains, said Heymach.
Intracranial Activity of Zongertinib
Heymach went on to say that high rates of brain metastases lead to poor outcomes in patients with HER2-mutant NSCLC9 and create the need for HER2-targeted therapies that demonstrate intracranial activity. Among 27 patients in Cohort 1 who were eligible for assessment by the Response Assessment in Neuro-Oncology Brain Metastases criteria, the confirmed intracranial ORR was 41% (95% CI: 25–59%) with a disease control rate of 81% (95% CI: 63–92%).8 The rate of confirmed systemic objective response in patients with brain metastases (64%) was comparable to that in the overall population (ORR: 71%), suggesting that zongertinib may provide clinical activity in patients with HER2-mutant NSCLC regardless of brain metastasis status, including in patients with central nervous system involvement.8
Consistent and Manageable Safety Profile
The safety profile of zongertinib was similar across all cohorts, with a low incidence of Grade ≥3 drug-related adverse events.8 In Cohort 1, 17% of patients experienced Grade ≥3 drug-related adverse events, with the most common being increased ALT levels (8%) and increased AST levels (5%).8 No cases of drug-related interstitial lung disease were reported in any cohort.8
The most common, any-grade drug-related adverse event in Cohort 1 was diarrhea, which occurred in 56% of patients; most events were Grade 1 (48%), and only 1% were Grade 3.8 In addition, all cases of drug-related rash (33%) were Grade 1 or 2.8 The low incidence of severe EGFR-related toxicities reflects the selective design of zongertinib, said Heymach. Only 7% of patients required dose reductions due to adverse events, and 3% discontinued treatment due to adverse events.8
Patient-reported Outcomes Indicate Symptom Improvement
Patient-Reported Outcome Assessment
An ASCO 2025 poster presentation included PROs from 30 participants in Cohort 1. PRO data included the 30-item European Organisation for Research and Treatment of Cancer-Quality of Life Group (EORTC QLQ-C30) physical functioning scale, NSCLC Symptom Assessment Questionnaire, and Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events items.10 PRO data were collected at multiple timepoints through Cycle 9, with high completion rates exceeding 86.7% across all visits.10
Sustained Functional Improvements
Mixed model repeated measures analysis demonstrated rapid improvements in both physical functioning and disease-related symptoms that were sustained throughout the assessment period.10 For EORTC QLQ-C30 physical functioning, the least squares mean change from baseline at Cycle 5 was 9.6 (95% CI: 6.3–12.9), and was maintained at Cycle 9 with 7.8 (95% CI: 3.2–12.5; Figure 2).10 Similarly, NSCLC Symptom Assessment Questionnaire total scores showed improvement, with a change from baseline of –3.9 (95% CI: -4.8– -2.9) at Cycle 5.10
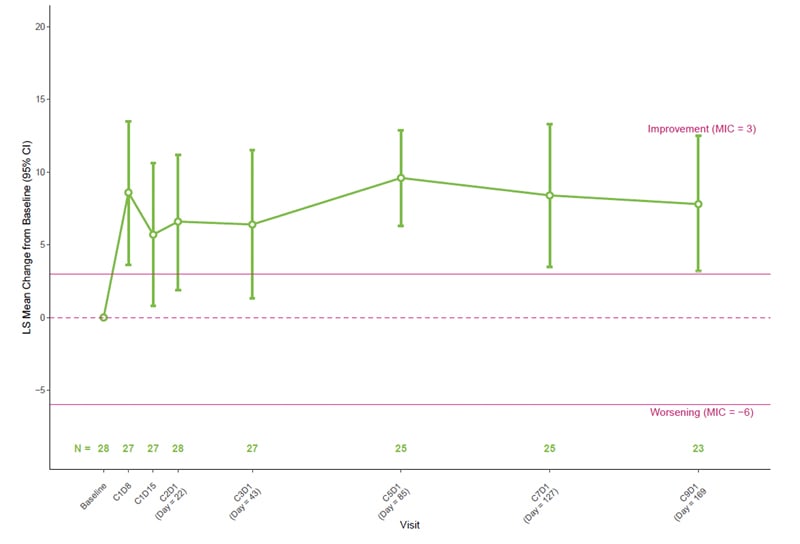
Figure 2: Least squares mean change from baseline in the European Organisation for Research and Treatment of Cancer-Quality of Life Questionnaire Core 30 physical functioning domain scores in patients with HER2-mutant non-small cell lung cancer treated with zongertinib (N=30).10
Higher scores represent better physical functioning. Rapid improvement was observed by Cycle 1 Day 8, with sustained benefits throughout treatment. At Cycle 5, the mean improvement was 9.6 points (95% CI: 6.3–12.9). Error bars represent 95% CI.
C: Cycle; D: day; LS: least squares; MIC: minimal important change.
Improvement in physical functioning peaked at Cycle 5, with 53.3% of patients experiencing meaningful improvement.10 Overall, physical functioning in over two-thirds of patients either improved or remained stable, with only a small number (3–5 patients) experiencing deterioration.10
Symptom-Specific Improvements
Individual symptom assessments revealed clinically meaningful improvements in disease-related symptoms. More than 50% of patients reported no coughing at Cycles 5 and 9, representing a doubling from baseline proportions.10 Dyspnea responses showed 30% and 10% increases in patients responding “never” or “rarely” at Cycles 5 and 9 compared to baseline, respectively.10
Favorable Tolerability Profile
The overall side effect burden remained low throughout treatment, with 80–90% of patients reporting being “not at all” or “a little” troubled by side effects across all visits.10 Patient-reported symptomatic adverse events aligned with the safety profile of zongertinib. While diarrhea was reported at the highest frequency, it was manageable in most cases.8,10 Approximately 50% of patients reported never experiencing diarrhea post-baseline, compared to 83.3% at baseline, and among those who did report diarrhea, the majority described it as occurring rarely or occasionally.10
Patient-Centered Outcomes
PRO data provide important context beyond traditional efficacy measures, demonstrating whether clinical benefits translate into functional improvements.11,12 Patients who showed stable disease as the best overall response after treatment with zongertinib experienced similar symptom improvements and functional stability compared to those achieving partial or complete responses, suggesting that clinical benefit extends beyond radiographic response criteria.10 These patient-centered outcomes may be particularly relevant in clinical practice, where quality of life considerations play an increasingly important role in treatment selection for patients with cancer.13-15
Conclusion
The data presented at AACR and ASCO 2025 support zongertinib as a promising treatment option for previously treated patients with HER2 (ERBB2)-mutant advanced NSCLC, a patient population that currently has limited therapeutic options. The combination of durable clinical activity, manageable safety profile, and meaningful patient-reported improvements addresses multiple dimensions of therapeutic benefit.8,10 The activity observed across different mutation types and in previously treated populations, including those who received HER2-directed ADCs, suggests a broad benefit within the HER2-mutant NSCLC population.8







