Abstract
Background: Abnormal uterine bleeding (AUB) affects 3–30% of perimenopausal women and 5–10% of those who are postmenopausal. While the aetiology of the condition in these women is mostly benign, it could also be a symptom of endometrial hyperplasia (EH) and endometrial cancer (EC). Conditions linked to EH and EC, such as obesity, diabetes, and hypertension, are on the rise in Kenya. Despite studies in various populations reporting differences in endometrial histopathology and the prevalence of EH and EC, data from sub-Saharan Africa remain scarce.
Objectives: To describe the clinical, ultrasound, and endometrial histopathological features; determine the prevalence of EH and EC; and identify factors associated with the diagnosis of the two conditions in peri- and postmenopausal women with AUB at the Moi Teaching & Referral Hospital (MTRH), a tertiary hospital in Eldoret, Kenya.
Methods: This was a cross-sectional study of women aged ≥40 years with AUB, conducted between June 2019–May 2020 at MTRH. A total of 64 perimenopausal women and 36 postmenopausal women were enrolled. Endometrial biopsies were performed using a Pipelle® (Cooper Surgical Inc., Trumbull, Connecticut, USA) endometrial suction curette, followed by histopathological evaluation by two pathologists. Data on sociodemographic, clinical and reproductive characteristics (BMI, chronic illness, bleeding patterns, parity, contraception use), and ultrasound findings (presence of uterine mass, endometrial thickness) were recorded. Associations between these characteristics and the diagnosis of EH/EC were tested using χ² or Fisher’s exact test, and significance was accepted at a p value ≤0.05.
Results: Of 64 perimenopausal women, 42 (65.6%) were either overweight or obese, and 11 (17.2%) had at least one chronic illness linked to EC. Of the 36 postmenopausal women, 31 (86.1%) were either overweight or obese, and 20 (55.5%) had at least one chronic illness linked to EC. From the perimenopausal group, 40 (62.5%) had cyclical or benign patterns, 23 (35.9%) had EH with or without atypia, and one (1.6%) had EC. EH without atypia was the most frequently seen individual pattern in this group (32.8%). Among the 36 postmenopausal women, 15 (41.7%) had cyclical or benign patterns, 14 (38.9%) had EH with or without atypia, and seven (19.4%) had EC. EH without atypia was also the most frequently seen individual pattern in this group (25.0%). None of the clinical and reproductive characteristics were significantly associated with the diagnosis of EH/EC. A thickened endometrium on ultrasound was the only feature associated with the diagnosis of EH/EC (p=0.004) on multivariate analysis.
Conclusion: The most frequently observed histopathological features in perimenopausal women with AUB and in women with postmenopausal bleeding at MTRH were normal cyclical patterns (secretory or proliferative) in the perimenopausal group, benign pathologies (chronic endometritis in perimenopausal and atrophic endometrium in postmenopausal), and EH without atypia in both groups of women. Only a small proportion of perimenopausal women were diagnosed with EH with atypia (3.1%) and EC (1.5%), the two pathologies of greatest clinical concern. Based on these findings, the authors do not recommend the routine performance of endometrial biopsies for perimenopausal women with AUB unless symptoms persist despite medical therapy and risk factor modification. Conversely, in women with postmenopausal bleeding, the authors strongly recommend histological evaluation of the endometrium, particularly in those with an endometrial thickness >4 mm on ultrasound, given the relatively high prevalence of EH with atypia (13.8%) and EC (19.4%) observed in this study.
Key Points
1. Endometrial histopathological patterns observed in perimenopausal women with abnormal uterine bleeding (AUB) in this study were predominantly normal cyclical patterns (39%), endometrial hyperplasia (EH) without atypia (32.9%), and benign endometrial pathologies, such as chronic endometritis (7.8%).2. Endometrial histopathological patterns observed in women with postmenopausal bleeding in this study were predominantly pathological: EH without atypia (25%), atrophic endometrium (19.4%), and endometrial cancer (19.4%).
3. While the overall prevalence of EH in perimenopausal women with AUB in this study was significantly high (35.9%), hyperplasia with atypia constituted <10% of these cases. Endometrial cancer in this group of women was seen even less frequently (1.6%). Due to the low prevalence of these two conditions, which are of greatest clinical concern, the authors do not recommend routine endometrial histological evaluation in this group of women unless AUB persists despite risk factor modification and medical therapy.
INTRODUCTION
Abnormal uterine bleeding (AUB) is defined as uterine bleeding that doesn’t correspond with the regularity, frequency, duration, or amount of blood flow of normal menstrual bleeding.1 Its prevalence among women of reproductive age varies widely, reported between 3–30%, with adolescents and perimenopausal women being the most affected. Postmenopausal bleeding is a subset of AUB, occurring in approximately 10% of postmenopausal women.2 Overall, a third of women will experience AUB at some point in their life, with as many as 70% of gynaecologic consultations in the perimenopausal and postmenopausal periods attributed to the condition.3
The aetiology of AUB in perimenopausal and postmenopausal women often includes ovulatory dysfunction or benign pathologies such as endometrial polyps, uterine leiomyomas, or endometrial atrophy. However, AUB could also be the first and principal symptom of more serious underlying conditions, such as endometrial hyperplasia (EH) and endometrial cancer (EC), particularly in higher-risk groups like perimenopausal or postmenopausal women. Endometrial biopsy is recommended in these higher-risk women to exclude precancerous or malignant changes, with the primary goal of detecting these changes early to facilitate timely interventions.4
EC is the leading gynaecologic malignancy in most developed countries, and the second most common malignancy after cervical cancer in developing countries.5,6 It predominantly affects women who are postmenopausal and perimenopausal, with only 2–5% of cases detected in women aged <40 years.7 Notably, AUB is the most frequently reported symptom in women with EC, and up to 90% of cases reported to have this symptom.8
While cervical cancer is the leading gynaecologic malignancy in Kenya, the true prevalence of EC remains less clearly defined. It is estimated that for every 30 cases of cervical cancer in the country, there is one case of EC. This highlights a significant yet under-recognised disease burden in our population.9 The rising prevalence of risk factors for EH and EC in Kenya, such as obesity, diabetes, and chronic hypertension, further reiterates the importance of understanding the regional epidemiology of the two conditions. Despite studies in various populations reporting differences in endometrial histopathology and the prevalence of EH and EC, data from sub-Saharan Africa remain scarce. This study aims to address these gaps by providing regional data on the sociodemographic, clinical, reproductive, ultrasound, and histopathological features of women with AUB in Western Kenya. The findings are intended to inform local clinical protocols for the investigation and management of AUB, with an emphasis on early detection of EH and EC.
METHODS
Study Design
This is a cross-sectional study that employed a census method for participant recruitment. Sampling was not applied as the estimated number of eligible patients was relatively low (an average of 110 patients per year, based on past records).
Study Setting
The study was conducted at the Gynecology Unit of Moi Teaching & Referral Hospital, Eldoret, Kenya, between the months of June 2019–May 2020. This tertiary hospital is the second largest national referral hospital in Kenya, serving a catchment population of approximately 20 million people. The Gynecology Unit comprises the Gynecology Out-Patient Clinic, Gynecology Ward, and the Gynecologic Oncology Ward.
Participants
All women aged ≥40 years, presenting with AUB (including women with postmenopausal bleeding), who met the eligibility criteria and consented, were recruited over a period of 12 months. There was no strict classification of perimenopause. The average age of entry into menopause in the Western Kenya region is 48 years, with most women taking around 2–8 years to transition into menopause. The authors thus categorised women aged ≥40 years who were presenting with AUB as perimenopausal (mean age: ~46 years), while those who were ≥40 years and were presenting with bleeding (postmenopausal bleeding) following at least 12 consecutive months of non-iatrogenic amenorrhoea were classified as postmenopausal (mean age: ~62 years)
- Inclusion criteria: women aged ≥40 years presenting with AUB.
- Exclusion criteria: women with positive pregnancy tests, overt vulvar/vaginal/cervical lesions, known bleeding disorders, or inability to undergo biopsy due to an obstructing intrauterine pathology or positioning issues.
Study Procedure
All women aged ≥40 years presenting with vaginal bleeding not characteristic of normal menses, or with postmenopausal bleeding, at the Gynecology Unit were approached and assessed for eligibility. A detailed history, physical examination, and routine investigations for AUB, including thyroid function tests (TFT), were ordered as indicated. Many participants had prior workups from lower-level facilities, including TFT. This was particularly true for the perimenopausal group. Due to resource limitations, the authors did not subject these participants to repeat testing for the sake of the study, except for those who were being seen for the first time. Needless to say, none of the participants who had thyroid function assessment had abnormal TFT results.
Pelvic ultrasound imaging was performed on all participants, followed by endometrial biopsy using a Pipelle® (Cooper Surgical Inc., Trumbull, Connecticut, USA) endometrial suction curette. Subsequent interviews were conducted using a pre-validated structured questionnaire after investigations, ultrasound imaging, and a successful endometrial biopsy procedure. This was done because part of the information being filled in the questionnaire comprised the ultrasound findings. Furthermore, since one of the exclusion criteria was the inability to undergo the biopsy procedure due to obstructing intrauterine pathology or positioning issues, the authors administered the questionnaire only to those who had a successful biopsy procedure.
Biopsy samples were promptly sent to the pathology department, where two expert pathologists independently conducted histological evaluations of each sample. Discrepant histopathological diagnoses were resolved through a joint review to reach consensus.
Data Collection and Variables
Data were collected using a pre-tested, interviewer-administered, structured questionnaire (Supplementary Figure 1), which captured the following data:
- Socio-demographic data: age, marital status, level of education, employment status, and medical insurance.
- Clinical characteristics: BMI, chronic illness (diabetes or chronic hypertension), cigarette smoking, and family history of cancer (breast, colon, ovarian or uterine cancer).
- Reproductive characteristics: menstrual status, age at menarche and menopause, current bleeding pattern (heavy, irregular, heavy and irregular, continuous, spotting), duration of AUB, hormonal contraceptive use (previous and/or current), and obstetric history (parity, age at first pregnancy).
- Pelvic ultrasound findings: uterine size, endometrial thickness (ET), and presence of uterine or endometrial mass.
The questionnaire was pre-validated during the first week of the study through an internal pre-test/pilot study. Connelly LM10 cites that “a pilot study sample can be 10% of the sample projected for the larger parent study.” Using this, the authors calculated the sample size for the pilot study from an estimated mean of 110 patients aged ≥40 years with AUB, arriving at 11 participants. The questionnaire was administered to 11 women with characteristics similar to the authors’ intended study population. Responses obtained from the 11 participants confirmed the clarity and suitability of the questionnaire for data collection, with the data obtained being adequate to answer the study’s primary objectives.
Consent acquisition, biopsy collection, and questionnaire administration were all carried out by this study’s principal investigator (Issa S. Moshey). Pelvic ultrasounds were done by the on-duty sonographer.
Statistical Data Analysis and Presentation
Descriptive statistics, such as the mean and the corresponding SD, were used to describe continuous variables such as age if Gaussian assumptions were met. Otherwise, the median and the corresponding interquartile range (IQR) were used. Gaussian assumptions were assessed using the Shapiro-Wilk test for normality. Non-continuous variables were described using frequencies and proportions. Associations between clinical, reproductive characteristics, and ultrasound findings, and the diagnosis of EH/EC were tested using the χ² or Fisher’s exact test, depending on cell counts. Significance was accepted at p value ≤0.05. Logistic regression was modelled using factors that were associated with EH/EC diagnosis in the bivariate analysis. Data were analysed using Stata 16, and results were presented using text and tables.
RESULTS
Between June 2019–May 2020, a total of 107 women aged ≥40 years who presented with either vaginal bleeding that was not characteristic of normal menses or postmenopausal bleeding were approached and assessed for eligibility. The participant recruitment process resulted in the recruitment of a total of 100 participants (64 perimenopausal and 36 postmenopausal). All 64 of the perimenopausal and 34 of the postmenopausal participants had adequate biopsy samples. The postmenopausal participants with inadequate samples each had repeat biopsies, which eventually yielded adequate specimens.
Sociodemographic Characteristics
The mean ages of the perimenopausal and postmenopausal groups were 46.05 years (SD: ±3.73) and 62.11 years (SD: ±7.96), respectively. The majority of the perimenopausal (65.6%) and postmenopausal (66.6%) women were married. Half of the perimenopausal and 47.0% of the postmenopausal participants had primary education as the highest level of education. The majority of the perimenopausal participants (67.2%) were employed, compared with only 44.4% of the postmenopausal group.
Clinical Characteristics
Out of the 64 perimenopausal women, 42 (65.62%) were either overweight or obese, and 11 (17.18%) had at least one chronic illness linked to EC (chronic hypertension, diabetes). Among the 36 postmenopausal women, 31 (86.11%) were either overweight or obese, and 20 (55.56%) had a history of at least one chronic illness linked to EC. Only one participant in the perimenopausal group (1.56%) and six in the postmenopausal group (16.67%) had a family history of a cancer linked to EC (breast, colorectal, ovarian, or uterine cancer). None of the participants reported cigarette smoking (active or passive).
Reproductive Characteristics
The mean menarcheal ages of the 64 perimenopausal and 36 postmenopausal women were 14.86 years (SD: ±1.53) and 14.78 years (SD: ±1.12), respectively. The mean age at menopause among the 36 postmenopausal women was 49.31 years (SD: ±5.0). The median parity was 4 (IQR: 3) for the perimenopausal group and 7 (IQR: 4) for the postmenopausal group.
In terms of bleeding patterns, 40 (62.50%) of the perimenopausal participants had heavy and irregular menses, 12 (18.75%) had heavy menstrual bleeding, and seven (10.94%) had non-stop bleeding as the most common bleeding patterns. In the postmenopausal group, 21 (58.34%) had spotting, nine (25.0%) had spotting alternating with heavy bleeding, and six (16.67%) had heavy bleeding alone. The median durations of AUB in the perimenopausal and postmenopausal groups were 21 months (IQR: 82) and 3 months (IQR: 11), respectively. Hormonal contraceptive use (current or past) was reported in 47 (73.44%) of the perimenopausal and 21 (58.33%) of the postmenopausal women.
Ultrasound Features
Out of the 100 perimenopausal and postmenopausal women, 92 (92%) underwent transabdominal ultrasound, while eight (8.0%) had transvaginal ultrasound. Among the 64 perimenopausal women, bulky uterus was reported in 33 (51.56%), hypoechoic uterine masses in 23 (35.93%), and endometrial thickening in 17 (26.56%). In the postmenopausal group, 15 participants (41.67%) were reported to have a bulky uterus, eight (22.22%) had a hypoechoic uterine mass, and 18 (50.0%) had endometrial thickening. ET thresholds considered abnormal were >16 mm in perimenopausal and >4 mm in postmenopausal women. In the postmenopausal group, only two out of seven (28.5%) women diagnosed with EC, all five (100%) diagnosed with EH with atypia, and eight out of nine (88.8%) diagnosed with EH without atypia had thickened endometrium. From the perimenopausal group, one patient diagnosed with EC, and only eight out of the 21 (38%) diagnosed with EH without atypia, had a thickened endometrium. Neither of the two women diagnosed with EH with atypia in this group had thickened endometrium.
Endometrial Histopathological Patterns of Perimenopausal and Postmenopausal Participants
Histological results in both groups of women were classified into three main categories: (1) normal cyclical patterns and benign pathologies group (N/B group); (2) EH with or without atypia group (EH group); and (3) malignancy group (M group).
Histopathological Patterns of Perimenopausal Participants
Out of 64 perimenopausal participants, 40 (62.5%) had normal/cyclical patterns or benign endometrial pathologies (N/B group), 23 (35.94%) had EH (EH group), and only one (1.56%) had EC (M Group). These data are presented in Table 1.
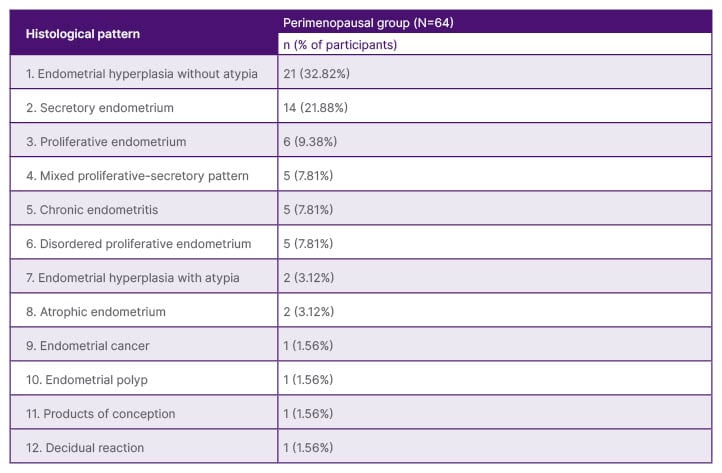
Table 1: Histopathological patterns of perimenopausal participants.
Histopathological Patterns of Postmenopausal Participants
Out of the 36 postmenopausal participants, 15 (41.66%) had N/B group patterns, 14 (38.89%) had EH group patterns, and seven (19.44%) had M group patterns (Table 2).
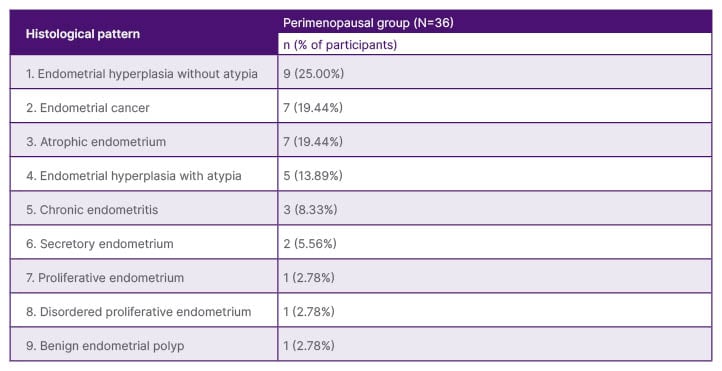
Table 2: Histopathological patterns of postmenopausal participants.
Clinical, Reproductive and Ultrasound Features Associated with Diagnosis of Endometrial Hyperplasia and Cancer
Out of the 100 biopsies done, 55 (55%) were N/B patterns, while 45 (45%) were EH and malignant (EC) pathologies. Data on factors linked to EH and EC, including clinical characteristics (BMI, chronic illness, family history of cancer, reproductive characteristics, and ultrasound features), of the 100 participants were stratified into the N/B and EH/EC groups to test for associations between these factors and a diagnosis of EH and cancer (EH/EC).
None of the clinical or reproductive characteristics were associated with a diagnosis of EH/EC on bivariate analysis (all p values >0.05).
When it comes to ultrasound features, endometrial thickening (p=0.001) and presence of hyperechoic complex mass/masses (p=0.014) were significantly associated with the diagnosis of EH and EC on bivariate analysis. Upon subjecting these two variables to a multivariate model, only endometrial thickening was established to be associated with the diagnosis of EH/EC (p=0.004; adjusted odds ratio: 4.17).
DISCUSSION
A number of studies globally have examined endometrial histopathological patterns in women with AUB. Many of these have focused on perimenopausal or postmenopausal women, while others have focused on all women, including those of reproductive age, <40 years. The authors’ study uniquely concentrates solely on perimenopausal and postmenopausal women, two groups of women with the highest incidence of EH and EC. Regional data on the subject remain limited, with most existing studies on the topic being from Northern and Western Africa.
The majority (65.63% of the perimenopausal and 86.11% of the postmenopausal participants) were either overweight or obese, which is comparable to findings by Elkholi et al.11 from Egypt, where all participants were either overweight or obese. In contrast, Meena et al.12 in India, reported that only 4% of their participants were overweight or obese. This difference could possibly be due to differences in age distribution, as their participants were relatively younger. Furthermore, the high rates of overweight and obesity among the authors’ participants reflect the country’s trend, as evidenced in a multiregional study by Mkuu et al.,13 which reported that one in three women (33%) in Kenya were either overweight or obese.13
Regarding chronic illnesses linked to EC, 26% of the participants in the authors’ study had chronic hypertension and 5% had diabetes. These findings are consistent with Elkholi et al.11 who reported a 20.6% prevalence of chronic hypertension in their participants. Comparable findings were also reported in India by Meena et al.,12 where 21% of the participants had chronic hypertension. In contrast, Piróg et al.14 from Poland reported a higher prevalence of hypertension and diabetes (50.9% and 17.5%, respectively).14 This difference reflects the higher burden of lifestyle-related non-communicable diseases in developed countries compared to developing countries.
The predominant bleeding pattern in the postmenopausal group in the authors’ study was spotting (55.56%), followed by heavy bleeding alternating with spotting (25%) and heavy bleeding alone (16.67%). This contrasts with the findings of Elkholi et al.11 from Egypt, who reported light menses-like bleeding as the most common pattern in women with postmenopausal bleeding (61.15%), followed by spotting (29.89%).11 The contrast could be due to the fact that they exclusively studied women who had an ET ≤4 mm, while in the authors’ study, all women with postmenopausal bleeding were included, regardless of their ET.
Cyclical endometrial patterns were a common feature in the authors’ perimenopausal participants. Interestingly, three postmenopausal women also exhibited cyclical patterns, despite these findings being uncommon after menopause due to the cessation of ovarian activity. The authors postulated that peripheral oestrogen sources (especially in women who are obese) or exogenous progestins could induce such proliferative or secretory changes in this group.
The authors acknowledge that EH with and without atypia are two different conditions with differing management strategies, with EH without atypia having a lower risk of progression (<5%) to EC compared to EH with atypia (8–29% risk), according to studies done in Western populations.15 However, the authors’ primary objective was to look at the prevalence of EH in general, and not just EH with atypia. The authors’ decision to do so aligns with the International Federation of Gynecology and Obstetrics (FIGO) classification system (PALM-COEIN) of AUB, where EH (both with and without atypia) falls under the M category that denotes ‘malignancy or EH’.
The prevalence of EH and EC in perimenopausal women in the authors’ study was 35.94% and 1.56%, respectively, comparable to findings by Abdelazim et al.16 from Egypt, who reported a prevalence of 35.70% of EH. However, the prevalence of EC in their study was higher compared to the authors’ study (6.40% versus 1.56%), which could be attributed to an older population in their study. Belcaro et al.17 from Italy reported a prevalence of 26.64% of EH in their study, somewhat comparable to the authors’ findings. However, the prevalence of EC in their study was remarkably lower than in this study (0.48% versus 1.56%).17 While the prevalence of EC in perimenopausal women in this study is comparable to that reported in similar studies from other regions, that of EH was relatively higher (Table 3).
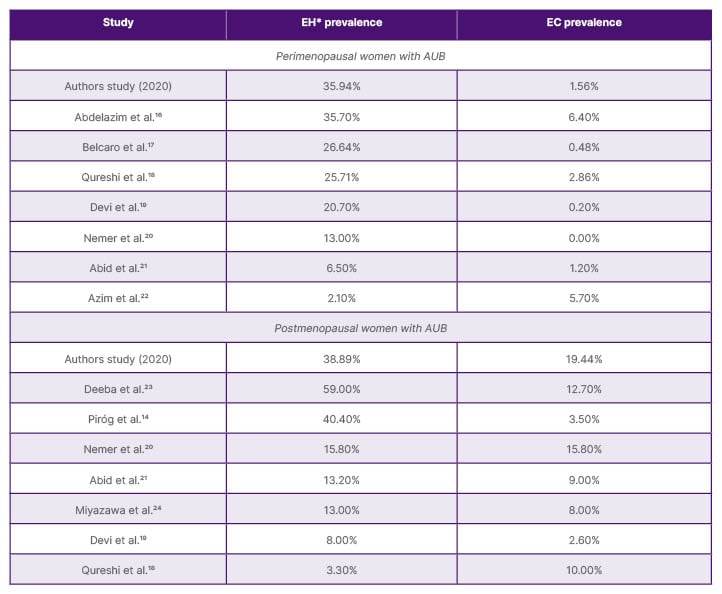
Table 3: Prevalence (%) of endometrial hyperplasia and cancer in perimenopausal and postmenopausal women with abnormal uterine bleeding reported in different studies.
*Both hyperplasia without atypia and with atypia combined.
AUB: abnormal uterine bleeding; EC: endometrial cancer; EH: endometrial hyperplasia.
The prevalence of EH and EC in postmenopausal women in this study was 38.89% and 19.44%, respectively, both markedly higher compared to the prevalences reported in other populations. Piróg et al.14 from Poland reported a prevalence of 40.40% of EH, which is comparable to the authors’ finding. However, the prevalence of EC in their study was much lower compared to the authors’ study (3.50% versus 19.44%), possibly due to their exclusion of women with ET >4 mm.14 Nemer et al.20 from Saudi Arabia, on the other hand, reported a lower prevalence of EH compared to the authors’ study (15.80% versus 38.89%) and a prevalence of EC that was comparable to the authors’ study (15.80% versus 19.44%).20 Overall, European studies showed lower EC rates in women with postmenopausal bleeding compared to studies from Asia and Africa (Table 3).
None of the clinical characteristics (BMI, diabetes, chronic hypertension, family history of significant malignancy, and reproductive characteristics) were associated with the diagnosis of EH/EC. This compares to findings by Piróg et al.14 from Poland, who reported that neither BMI, diabetes, nor chronic hypertension was associated with the diagnosis of EH/EC in women with postmenopausal bleeding.14 However, numerous systematic reviews have established an association between BMI, diabetes, chronic hypertension, and the diagnosis of EH/EC.25-27 This discrepancy may stem from the authors’ limited sample size and statistical power to test for associations.
In this study, ET cut-offs of 16 mm for perimenopausal and 4 mm for postmenopausal women were used to define a thickened endometrium. In premenopausal women, ET normally fluctuates throughout the menstrual cycle, ranging from 1–16 mm (1–4 mm immediately after menstruation, 5–7 mm in the early proliferative phase, 8–11 mm in the late proliferative phase, and 7–16 mm in the secretory phase). Given this variability, the use of a fixed ET threshold in premenopausal women can be challenging due to a significant overlap between normal and abnormal values. To address this, the authors classified any ET measurement exceeding 16 mm (the uppermost limit observed during any phase of the menstrual cycle) as abnormal or thickened. This approach was particularly appropriate for the authors’ perimenopausal participants, many of whom had irregular or erratic cycles, making it difficult to determine the specific phase of the menstrual cycle they were in at the time of ultrasound evaluation.
From the ultrasound findings, ET was the only factor significantly associated with the diagnosis of EH/EC in both bivariate and multivariate analyses in this study. This compares to findings by Piróg et al.,14 who reported that EH/EC were unlikely to be present in women with postmenopausal bleeding who had an ET <4 mm. ET measurement is also applied in predictive tools for the assessment of EC risk in women with AUB, particularly postmenopausal women, e.g., the risk of EC scoring model.28
CONCLUSION
This study revealed a relatively higher prevalence of EH in perimenopausal women, and both EH and cancer in postmenopausal women with AUB, compared to most studies from other populations. Nevertheless, the most frequently observed histopathological features were normal cyclical patterns (secretory or proliferative) in the perimenopausal, benign pathologies (chronic endometritis in perimenopausal, and atrophic endometrium in postmenopausal), and EH without atypia in both groups. Only a small proportion of perimenopausal women were diagnosed with EH with atypia (3.1%) and EC (1.5%), the two pathologies that are of greatest clinical concern. Based on these findings, the authors do not recommend the routine performance of endometrial biopsies for perimenopausal women with AUB, unless their symptoms persist despite medical therapy and risk factor modification. Conversely, in women with postmenopausal bleeding, the authors strongly recommend the performance of endometrial histological evaluation, particularly in those with an ET >4 mm on ultrasound, given the relatively high prevalence of EH with atypia (13.8%) and EC (19.4%) observed in this study.
Study Strengths
To the best of the authors’ knowledge, this study was the first of its kind in sub-Saharan Africa at the time it was conducted, providing insights into an important subject that could be used by others from the region to explore more areas of the topic.
All endometrial biopsies were taken by one qualified person, with an overall sample adequacy rate of 100%.
Histological evaluation of all biopsies was done by two expert consultant pathologists to minimise inter-observer variability.
Study Limitations
There is a relatively lower accuracy of the Pipelle endometrial suction curette in detecting focal pathologies such as endometrial polyps and fibroids compared to hysteroscopically guided biopsies.
The pelvic ultrasounds performed in this study were mostly transabdominal and not transvaginal, which has a higher accuracy in the detection of pelvic pathologies and measurement of ET. The ultrasounds were also performed by different sonographers depending on their availability at the time the patient was being seen.
The authors note that, with the study having been done at a tertiary hospital, its findings may have been affected by referral bias and may also not be generalised to the population.