| Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App store. Adverse events should also be reported to Theramex on [email protected] or Tel: +44 (0)333 009 6795. |
Meeting Summary
Uterine fibroids are benign smooth muscle tumours occurring in the uterus. Although some women are able to live with uterine fibroids without treatment, the condition can be extremely debilitating for many causing heavy menstrual bleeding, pain, and a substantial reduction in quality of life (QoL). Treatment for uterine fibroids has traditionally been sub-optimal, with substantial drawbacks associated with current surgical, non-surgical, and medical treatments. The recent availability of gonadotropin-releasing hormone (GnRH) antagonists such as linzagolix for the management of uterine fibroids may offer alternative treatment options for women affected by this debilitating condition. This article summarises a Theramex-sponsored symposium entitled ‘Linzagolix – a flexible treatment option for women with uterine fibroids’, delivered on 18th April 2024 as part of the Society of Endometriosis and Uterine Disorders (SEUD) Congress programme in Geneva, Switzerland. The symposium had the participation of a distinguished panel of experts who presented the current and evolving landscape in the management of uterine fibroids. Francisco Carmona, SEUD President and former Head of the Gynaecology Department, Clinic Institute of Gynaecology, Obstetrics and Neonatology (ICGON), Hospital Clinic of Barcelona, Spain, emphasised the considerable burden of uterine fibroids on individual women and society as a whole; while Sven Becker, Director of the Department of Obstetrics and Gynaecology at Frankfurt University’s Medical Centre, Germany, presented data from the PRIMROSE trials of linzagolix, a treatment that offers flexible, individualised therapy for women with uterine fibroids. The symposium was chaired by Marie-Madeleine Dolmans, SEUD board member and Head of Department at Cliniques Universitaires Saint Luc, Brussels, Belgium, who also introduced and concluded the meeting.Introduction
Dolmans began by reminding the audience that uterine fibroids are benign smooth muscle tumours in the uterus, which often do not require treatment.1,2 She explained that a diagnosis of fibroids is based on clinical assessment, ultrasound, or histological examination of hysterectomy specimens with a prevalence estimate ranging widely depending on study populations and diagnostic methods.3 While the exact aetiology of fibroids remains unknown, it has been established that hormones (especially oestrogen and progesterone) play a key role by modulating the expression of growth factors that are implicated in cell proliferation.2,4
A cross-sectional survey conducted in Western Europe revealed a prevalence of diagnosed uterine fibroids of 11.7–23.6% across France, Germany, Italy, Spain, and the UK, with an average age at diagnosis of around 40 years.5 Dolmans noted that approximately one-third of cases of uterine fibroids remain undiagnosed for 5 years or more, possibly because of the symptoms being misdiagnosed as heavy menstrual bleeding. She also highlighted the high proportion (between 24.1–57.4%) of patients who do not receive any treatment for the condition.5
Dolmans observed that many fibroids are asymptomatic and are often only diagnosed by chance. However, in around 30–40% of cases, patients may experience a number of unpleasant symptoms, including heavy menstrual bleeding, iron deficiency, and anaemia, as well as pain and bulk-related symptoms such as bowel and bladder dysfunction, increased daytime urinary frequency, and urinary incontinence.6 Such symptoms may have a detrimental effect on the patient’s overall QoL.6
Treatments for uterine fibroids broadly fall into three categories: surgery, non-surgical alternatives, and medical therapy.6 The most appropriate treatment modality is selected based on specific clinical characteristics such as number, size, and location of the fibroids, as well as patient factors, including age and desire to preserve fertility and avoid hysterectomy.6 However, despite the availability of a number of treatment options for the management of uterine fibroids, several unmet clinical needs remain, including the need for low-risk, cost-effective, fertility-sparing medical options, and an emphasis on the prevention or early treatment of fibroids.7 Dolmans observed that one of the aims of the current meeting was to address these outstanding issues, and introduced Carmona to discuss the disease burden of uterine fibroids, as well as possible future directions for treatment.
Uterine Fibroids: Disease Burden and Future Directions
Carmona reiterated the impact of uterine fibroids on patient QoL by describing a literature review of 40 studies that reported significantly lower health-related QoL scores across all measures for women with uterine fibroids compared with those without this disorder.8 One particularly notable impact of uterine fibroids is on reproductive dysfunction, where uterine fibroids are the primary cause of infertility in 1–3% of patients,9 with decreased pregnancy and implantation rates and increased pregnancy loss seen with submucosal and intramural fibroids, respectively.10
Current management of uterine fibroids continues to rely largely on costly surgical procedures, with around 200,000 fibroid-related hysterectomies carried out each year in the USA alone.11 However, satisfaction with current non-surgical techniques is poor, leading many women to opt ultimately for major surgery despite its cost and invasive nature.11 Carmona explained that there is a clear need for therapeutic alternatives that aim to shrink fibroids, control bleeding, minimise adverse events, treat anaemia, demonstrate cost effectiveness, and (most importantly) enhance QoL. He also emphasised the importance of tailoring treatment to the needs of individual women, taking into account symptoms and patients’ desire to preserve their fertility and uterus, and avoid radical surgery such as hysterectomy.6
A number of medical alternatives to surgery are available for the management of uterine fibroids; however, none of the current options are without significant drawbacks. For example, progestogens have been used for many years for the management of uterine fibroids, despite the lack of evidence and absence of adequately designed and powered studies. A recent review of the available literature on progestogen therapy found that, overall, there is a lack of evidence of efficacy, and that progestogens may even increase the size of uterine fibroids.12
Similarly, although tranexamic acid may reduce heavy menstrual bleeding in women without uterine fibroids, its impact on patients with uterine fibroids is unknown, and its use is contraindicated in women with active thromboembolic disease or a history of thrombosis.13 Use of intrauterine devices has also been suggested for the treatment of uterine fibroids. However, while this option has been shown to significantly reduce menstrual bleeding, it cannot be used if the uterine cavity is distorted by fibroids.11 In addition, high expulsion rates have been reported in women with submucosal fibroids.11
GnRH agonists (such as leuprolide acetate) may be given for 3–6 months before surgery to reduce fibroid size and correct iron deficiency anaemia.11 However, extended treatment beyond 6 months has been shown to reduce bone mineral density (BMD), as well as increase vasomotor and other menopausal symptoms, and long-term use of this therapy is contraindicated.11 Selective progesterone receptor modulators such as ulipristal acetate have been shown to reduce fibroid volume, achieve a marked and rapid control of bleeding, and restore haemoglobin levels.11 However, due to rare cases of liver toxicity, the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) has significantly restricted the approved indication and usage of ulipristal acetate in the treatment of uterine fibroids.11
GnRH receptor antagonists are the most recent medical treatment approaches approved for the treatment of uterine fibroids, and are now available in many countries around the world.14 Elagolix was initially approved in the USA in July 2018 for the management of moderate-to-severe pain associated with endometriosis, and then for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women in May 2020.14,15 Relugolix has been approved in the USA since May 2021 for the management of heavy menstrual bleeding associated with fibroids, and is now approved in the EU and UK for treatment of moderate-to-severe symptoms of uterine fibroids in women of reproductive age, as well as for the symptomatic treatment of endometriosis in women with a history of previous medical or surgical treatment for their endometriosis.16,17 Most recently, linzagolix has been approved in the EU and UK for the treatment of moderate-to-severe symptoms of uterine fibroids in adult women of reproductive age.18
Carmona went on to describe the mechanism of action of linzagolix, explaining how the drug binds competitively to GnRH receptors in the pituitary gland to prevent receptor activation by endogenous GnRH. This leads to a rapid suppression of luteinising hormone and follicle-stimulating hormone, resulting in a dose-dependent decrease in serum oestradiol concentration.19-21 He noted that the clinical benefits of linzagolix include its oral delivery, rapid reversibility, and immediate gonadotropin suppression without flare effect, as well as dose-dependent partial or full oestrogen suppression.19-21
Linzagolix may be administered with or without hormonal add-back therapy (ABT) in order to maintain oestradiol concentrations within the optimal range of 20–60 pg/mL.18,19,21 At a dose of 200 mg, linzagolix achieves full suppression of oestradiol, leading to a higher incidence of hot flushes and a greater risk of BMD loss.20 Concomitant administration of ABT with 200 mg linzagolix has the effect of returning oestradiol levels to the optimal range. In contrast, at a dose of 100 mg, linzagolix achieves only partial suppression, stabilising oestradiol levels within the optimal zone and eliminating the need for ABT.18,19
Use of ABT in conjunction with linzagolix is associated with a number of benefits and risks, which must be balanced to ensure an individualised approach based on the patient’s clinical profile and baseline risk. Examples of the benefits of ABT therapy include amelioration of hypoestrogenic symptoms and the prevention of excessive loss of BMD. However, balanced against this are the increased risk of thromboembolic disorders, vascular events, and oestrogen-related malignancy.22 Furthermore, ABT is not appropriate for all women as some may prefer to avoid hormonal treatments altogether, or have contraindications for ABT such as venous thromboembolic disorders, arterial thromboembolic cardiovascular diseases, or oestrogen-related malignancies amongst others.
Carmona concluded his presentation by reminding the audience of the high unmet need that persists for pharmacological treatments that are able to reduce fibroid size for symptomatic women who wish to preserve fertility. He also noted that treatment guidelines need updating to include the GnRH antagonist class in the treatment of women with uterine fibroids, especially in those who are unsuitable for treatment with existing therapies, or who may wish to avoid invasive surgeries such as myomectomies or hysterectomies. Finally, he observed that linzagolix can be used with or without ABT, offering flexible dosing for the personalised treatment of uterine fibroids.
Linzagolix: The Option of Flexible Individualised Therapy for Women with Uterine Fibroids
Becker opened his presentation on the PRIMROSE trial programme by reminding the audience that linzagolix is approved in Europe and the UK for the treatment of moderate-to-severe symptoms of uterine fibroids in adult women of reproductive age.18 He also welcomed the fact that linzagolix is an oral treatment that offers flexibility in terms of dosing and concomitant use of ABT, allowing for a truly individualised treatment for patients with uterine fibroids.18 Becker’s presentation focused on results of the two Phase III PRIMROSE trials, evaluating the effect of linzagolix with and without hormonal ABT for the treatment of patients with symptomatic uterine fibroids.20
PRIMROSE 1 (NCT03070899) and PRIMROSE 2 (NCT03070951) were virtually identical, 52-week treatment, randomised, double-blind, placebo-controlled Phase III trials involving 1,012 patients overall. PRIMROSE 1 was carried out in the USA, whilst PRIMROSE 2 was performed primarily in Europe.20 Participants eligible for the PRIMROSE trials were women aged 18 years or over with at least one ultrasound-confirmed fibroid 2–12 cm in diameter or multiple small fibroids with a calculated uterus volume of more than 200 cm³, as well as heavy menstrual bleeding (defined as ≥80 mL menstrual blood loss per cycle) for at least two cycles. Women were excluded if they were pregnant, breastfeeding, or planning a pregnancy, had only subserosal pedunculated fibroids, history of systemic glucocorticoid therapy usage, were at substantial risk of osteoporosis, had abnormal hepatic function, severe coagulation disorder, or haemoglobin levels <6 g/dL.20
Patients in both PRIMROSE trials were randomised to placebo or one of four linzagolix dosing regimens (Figure 1).20 Becker explained that four active treatment groups were included to ensure the maximum amount of data about the effects of this treatment could be obtained. The primary efficacy endpoint was menstrual blood loss ≤80 mL and ≥50% reduction from baseline in the 28 days before week 24. Key secondary endpoints assessed at Week 24 included time to reduced menstrual blood loss, rates of amenorrhoea, changes in haemoglobin levels in anaemic patients, pain related to uterine fibroids, uterine and fibroid volume, and QoL.20
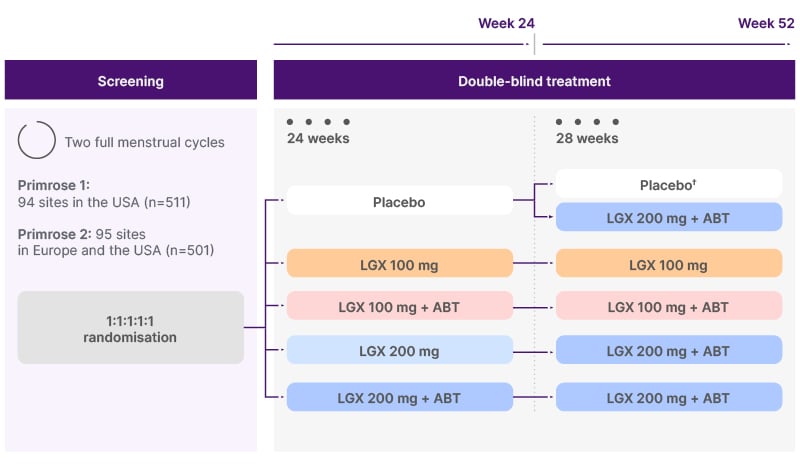
Figure 1: PRIMROSE study design.20
†In PRIMROSE 1, half of the women on placebo (n=31) continued placebo until 52 weeks.
ABT: add-back therapy (1 mg estradiol + 0.5 mg norethisterone acetate); LGX: linzagolix.
A review of the baseline characteristics of patients enrolled in the PRIMOSE trials revealed that the mean age of enrolled patients ranged from 41.3–43.4 years, which is typical for patients with uterine fibroids. In the USA population of PRIMROSE 1, there was a large proportion of patients of African descent (61–65%) as compared with the primarily European population of PRIMROSE 2 (4–6%).20 Becker noted that women of African descent have a much higher disposition toward fibroids, including very large fibroids. He also observed that the BMI of patients tended to be higher in PRIMROSE 1 than PRIMROSE 2 (32.2–33.3 versus 26.8–27.4 kg/m2), and that mean baseline haemoglobin level was low across all groups in both studies (10.5–11.7 g/dL).20
Analysis of the primary endpoint showed that linzagolix significantly reduced heavy menstrual bleeding in both the 100 mg and 200 mg dose groups, with and without ABT (all p≤0.003).20 This effect was significantly larger in both 200 mg dose groups compared with placebo and all other treatment groups, and was also significantly greater when linzagolix was administered in conjunction with ABT in each case (Figure 2).20
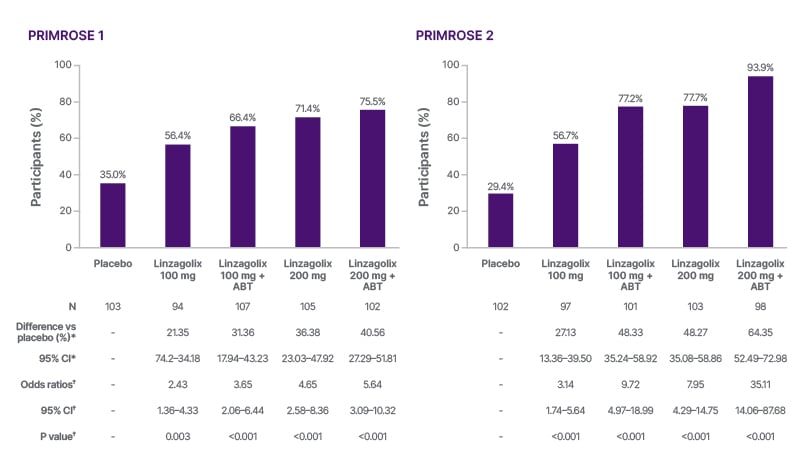
Figure 2: Primary endpoint: proportion of women with a reduced menstrual blood loss of ≤80 mL and ≥50% reduction from baseline at 24 weeks (PRIMROSE 1 and PRIMROSE 2).20
Each active treatment group was compared with the placebo (p=0.0125 or less was considered statistically significant).
*Common risk (proportion) difference between each linzagolix group and placebo, with race as a stratification factor, along with stratified Newcombe confidence limits.
†Cochran–Mantel–Haenszel test with race as a stratification factor.
ABT: add-back therapy; vs: versus.
Figure adapted from Donnez et al.20
Time to reduced menstrual blood loss was improved at 24 weeks in all linzagolix treatment groups, both with or without ABT compared to placebo.20 Notably, the time to heavy menstrual bleeding reduction was significantly shorter with linzagolix treatment, with a median time to response of just 3 days in the 200 mg groups, irrespective of ABT use (p<0.001).23 Over the long term, the reduction in heavy menstrual bleeding was maintained to the end of the study (52 weeks), even for those patients receiving 100 mg linzagolix without ABT.20
Compared with placebo, linzagolix 100 mg and 200 mg with or without ABT significantly increased the proportion of women with amenorrhea, to up to 81% of women in the linzagolix 200 mg group with ABT.20 Becker noted that amenorrhea is considered by many women to be a significant advantage of treatment. There was also a significant change in fibroid volume at 24 weeks in the linzagolix 200 mg group without ABT in both trials. In this treatment group, a 43% and 49% reduction in fibroid volume was observed in PRIMROSE 1 and PRIMROSE 2, respectively,20 showing that suppression of serum oestradiol to a concentration below 20 pg/mL is needed to achieve the greatest impact on fibroid volume.20 Indeed, mean fibroid volumes increased towards baseline volumes when ABT was added after 6 months of treatment with linzagolix 200 mg without ABT.20 Haemoglobin levels in anaemic subjects were also significantly improved at Week 24 for all treatment arms (p<0.001), except for haemoglobin concentrations in the 100 mg group without ABT in PRIMROSE 1 (p=0.019).20 With regards to pain reduction, by 24 weeks, between 28–54% of women across all linzagolix treatment groups reported a reduction in pain score of at least two categories (measured on a numeric rating scale NRS from 0 [no pain] to 10 [worst possible pain]), compared with 9–15% of placebo patients.20 Health-related QoL total score was also substantially higher for all linzagolix groups at Week 24 compared with placebo, and the effect was maintained for all groups up to 52 weeks.20
In relation to the PRIMROSE 1 and 2 trials safety data, between 44–67% of women reported at least one adverse event (AE) up to Week 24 across the PRIMROSE trials in the linzagolix groups compared with 45–54% in the placebo groups.20 The incidence of AEs was slightly higher among women taking linzagolix alone compared with linzagolix plus ABT. The most common AE was hot flushes. Across both trials, 2% of women experienced serious AEs. Between Weeks 24 and 52, 43% and 30% of women reported at least one AE in PRIMROSE 1 and PRIMROSE 2, respectively, with no clear pattern of incidence between groups.20 There was a low rate of hot flushes across all groups between Weeks 24–52 (0% in PRIMROSE 1 and 1% in PRIMROSE 2).20
In terms of lumbar spine BMD, relatively small dose-dependent decreases of up to 4.1% with 200 mg linzagolix and 2% with 100 mg linzagolix were observed at 24 weeks, with the effect attenuated by ABT.20 Continued small BMD decreases of up to 2.4%, 1.5%, and 2% were observed at 52 weeks for 100 mg linzagolix, 100 mg linzagolix with ABT, and 200 mg linzagolix with ABT, respectively.20 The Z-score compares BMD to the average values of a person of a similar age and gender, with a score of less than −2 indicating lower bone mass than expected. Analysis of the Z-scores of the lumbar spine at baseline and 24 and 52 weeks showed that the bone health of women in the PRIMROSE trials was comparable to women of the same age without fibroids, and that the median and interquartile range of the Z-scores did not meaningfully change over time (Figure 3).20
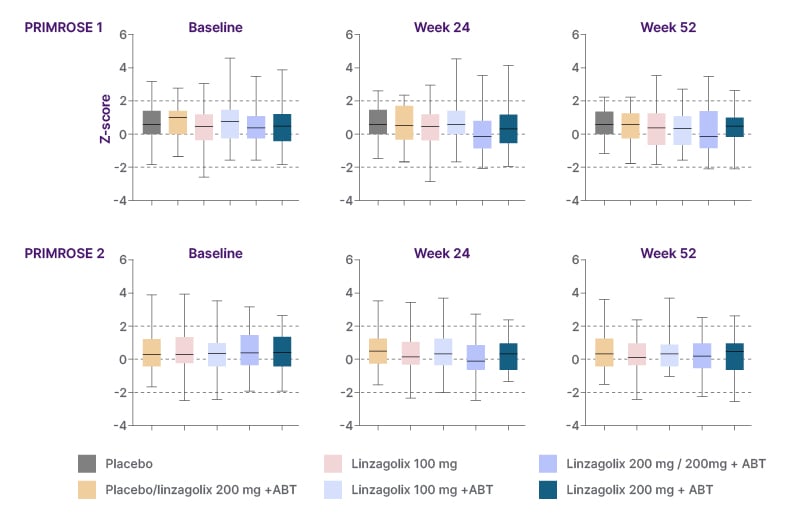
Figure 3: Z-scores of the lumbar spine at baseline, 24 weeks, and 52 weeks.
Z-scores compare BMD to the average values of a person of the same age and gender. Error bars are 95% CI.
A score <-2 is a sign of less bone mass than expected.
Women allocated to placebo or 200 mg linzagolix alone were switched to 200 mg linzagolix with ABT after 24 weeks, except in PRIMROSE 1, in which 50% of the women on placebo continued placebo (selected at random assignment) until Week 52.
ABT: add-back therapy; BMD: bone mineral density; LGX: linzagolix.
Adapted from Donnez et al.20
Conclusion
By the end of this symposium, delegates had been reminded of the ongoing unmet need for pharmacological treatments that are not only able to rapidly control symptoms of heavy menstrual bleeding, but also preserve their fertility, reduce their fibroid size, and enhance the QoL of women suffering from uterine fibroids. The GnRH receptor antagonist linzagolix is now approved in Europe and the UK for the treatment of moderate-to-severe symptoms of uterine fibroids in adult women of reproductive age.18 However, the guidelines still need to be updated to include the GnRH antagonist class in the treatment of women with fibroids who are not suitable for existing therapy or may wish to avoid surgery.
The recent PRIMROSE trials evaluating the effect of linzagolix with and without hormonal ABT for the treatment of patients with symptomatic uterine fibroids showed a significant reduction of heavy menstrual bleeding within 3 days of initiating linzagolix therapy at a dose of 200 mg with or without ABT.23 The studies also showed that linzagolix reduced fibroid volume by 43–49%,20 and that up to 42% of linzagolix-treated women with moderate-to-severe pain (numeric rating scale ≥4) had a reduction in pain level to minimal or no pain, while health-related QoL total score was substantially higher for all linzagolix doses. Thus, the availability of linzagolix provides additional treatment options for women with uterine fibroids, offering more choice, greater flexibility, and the possibility of more individualised treatment.
Yselty® (Linzagolix)▼ Prescribing Information
Please consult the Summary of Product Characteristics for other adverse reactions and full prescribing information before prescribing.
Presentation: Yselty 100 mg and Yselty 200 mg film-coated tablet. Indication: Yselty is indicated in adult women of reproductive age for the treatment of moderate to severe symptoms of uterine fibroids, and for symptomatic treatment of endometriosis in women with a history of previous medical or surgical treatment for their endometriosis. Dosage and administration: For oral use. Yselty can be taken with or without food. The 200 mg dose can be taken as either one 200 mg tablet or two times a 100 mg tablet. Yselty treatment should be initiated and supervised by a physician experienced in the diagnosis and treatment of uterine fibroids and/or endometriosis. Pregnancy must be ruled out prior to initiating treatment. Yselty should preferably be started in the first week of the menstrual cycle and should be taken continuously once daily. In patients with risk factors for osteoporosis or bone loss, a dual X-ray absorptiometry (DXA) scan is recommended prior to starting Yselty treatment. Yselty can be taken without interruption. A DXA scan is recommended after 1 year of treatment for all women, and there is a need for continued Bone Mineral Density (BMD) monitoring thereafter. The recommended dose is: For Uterine Fibroids: 100 mg or, if needed, 200 mg once daily with concomitant hormonal add-back therapy (ABT, estradiol 1 mg and norethisterone acetate 0.5 mg tablet once daily); 100 mg once daily for women in whom ABT is not recommended or who prefer to avoid hormonal therapy; 200 mg once daily for short-term use (< 6 months) in clinical situations when reduction of uterine and fibroid volume is desired. Fibroid size may increase when the treatment is stopped. Due to the risk of BMD decrease with prolonged use, the 200 mg dose without concomitant ABT should not be prescribed for longer than 6 months. For Endometriosis: 200 mg once daily with concomitant hormonal add-back therapy. Missed doses: If a dose is missed, treatment must be taken as soon as possible and then continued the next day at the usual time. Contraindications: Hypersensitivity to the active substance(s) or to any of the excipients list, Pregnancy or breast-feeding, Known osteoporosis, Genital bleeding of unknown aetiology, Contraindications related to ABT should be respected if concomitant ABT is given. Special warnings and precautions for use: Medical examination/consultation: Prior to the initiation or reinstitution of Yselty, a complete medical history (including family history) must be taken. Blood pressure must be measured, and a physical examination must be performed guided by the contraindications and warnings for use. During treatment, periodic check-ups must be carried out according to standard clinical practice. Any hormonal contraception needs to be stopped prior to initiation of Yselty. Pregnancy must be ruled out prior to administering or re-initiation of Yselty. Risk of bone loss: In some women treated with Yselty, who had normal BMD at start of treatment, BMD loss varying from > 3-8% was reported. The benefits and risks of Yselty in patients with a history of a low trauma fracture or other risk factors for osteoporosis or bone loss, including those taking medications that may affect BMD should be considered prior to initiating treatment. It is recommended to perform a dual X ray absorptiometry (DXA) scan before commencing treatment with Yselty in these at-risk patients. A DXA scan is recommended after 1 year of treatment for all women to verify that the patient does not have an unwanted degree of BMD loss. Thereafter, depending on the prescribed dose of Yselty, BMD assessment is recommended annually (Yselty 100 mg) or at a frequency determined by the treating physician based on the woman’s individual risk and previous BMD assessment (Yselty 100 mg with concomitant ABT and Yselty 200 mg with concomitant ABT). If the risks of BMD decrease exceed the potential benefit of treatment with Yselty, treatment should be discontinued. Hepatic impairment: No dose adjustment is necessary in women with mild or moderate hepatic impairment. Yselty should be avoided in women with severe hepatic impairment. Renal impairment: Yselty should be avoided in women with moderate (eGFR = 30-59mL/min), severe renal impairment (eGFR < 30mL/min) or end-stage renal disease. Prescribers are recommended to monitor for adverse reactions in women who have mild renal impairment (eGFR = 60-89 mL/min) although no dose adjustment is required. Paediatric population: There is no relevant use of Yselty in children aged under 18 years for the indication of treatment of moderate to severe symptoms of uterine fibroids. The safety and efficacy of Yselty in children aged under 18 years for the indication of treatment of endometriosis has not been established. Cardiovascular disorders/QT prolongation: Yselty marginally increases the QT interval but showed no evidence of clinically relevant risk of QT prolongation or Torsade de Pointes. Caution should be exercised in patients who have known cardiovascular disease, family history of QT prolongation or hypokalaemia, and in concomitant use with medicinal products known to prolong the QT interval. Caution should also be exercised in patients with co-existing disorders leading to increased Yselty plasma levels. Contraception: Linzagolix with or without concomitant ABT has not been demonstrated to provide contraception. Women of childbearing potential at risk of pregnancy have to use effective non-hormonal contraception whole on treatment with Yselty. Change in menstrual bleeding pattern and reduced ability to recognise pregnancy: Women should be informed that treatment with Yselty usually leads to a significant reduction in menstrual blood loss and often leads to amenorrhoea, which may reduce the ability to recognise the occurrence of a pregnancy in a timely manner. Pregnancy testing should be performed if pregnancy is suspected, and treatment should be discontinued if pregnancy is confirmed. Liver enzymes: Asymptomatic transient liver enzyme elevations have been reported. Patients should be instructed to promptly seek medical attention in case of symptoms or signs that may reflect liver injury, such as jaundice. Treatment should be discontinued if jaundice develops. Acute liver test abnormalities may necessitate discontinuation of treatment with Yselty until liver tests return to normal. Women with abnormal hepatic function parameters (≥2 upper limit of normal, ULN) were excluded from studies with Yselty. Therefore, in women with known abnormal hepatic history, a baseline level of hepatic function tests should be obtained, and further regular monitoring should be performed. These patients should be treated with caution. Lipid levels: Increases in lipid levels were observed with Yselty treatment. These increases were generally of no clinical relevance. However, in women with pre-existing elevated lipid profiles monitoring of lipid levels is recommended. Mood disorders: Mood disorders including depression, alterations in mood, and emotional lability have been observed with treatment with GnRH antagonists. Caution is to be applied in women with a history of depression and/or suicidal ideation. Patients with known depression or history of depression should be carefully monitored during treatment. Treatment should be discontinued if depression recurs to a serious degree. Warnings and precautions relevant to ABT. If concomitant ABT is prescribed, all warnings and precautions relevant to ABT should be considered. Lactose: Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product. Interactions: CYP2C8 substrate medicinal products, Yselty has been shown to increase mean repaglinide (a CYP2C8 sensitive substrate) exposure in healthy subjects by less than 2-fold. Due to the risk of increased plasma concentrations, concomitant administration of Yselty and medicinal products primarily cleared by CYP2C8 metabolism and with a narrow therapeutic index should be avoided. Prescribers are recommended to monitor for increases in adverse reactions associated with other CYP2C8 substrates when co-administered with Yselty. Fertility, pregnancy and lactation: Linzagolix with or without ABT has not been demonstrated to provide contraception. Women of childbearing potential at risk of pregnancy have to use effective non-hormonal contraception while on treatment with Yselty. Yselty is contraindicated during pregnancy and breast-feeding. Side effects: The most common adverse reactions reported in the pivotal phase 3 clinical studies in the uterine fibroid-treated population were hot flushes and headaches, which were reported with higher frequency at higher doses and less frequently when ABT was taken concomitantly. Hot flushes were reported in 5.2%, 9.6%, 10.1% and 31% of women treated with 100 mg with ABT, 200 mg with ABT, 100 mg and 200 mg, respectively. Similarly, headaches were reported more frequently at higher doses and decreased with ABT (1.4%, 2.4%, 4% and 6.2% for 100 mg with ABT, 200 mg with ABT, 100 mg and 200 mg, respectively). The most common adverse reactions reported in the endometriosis population treated with the recommended dose of 200 mg with ABT included hot flushes (6.3%) and headache (5.7%). Very Common (≥1/10): Hot flush; Common (≥1/100 to <1/10): Mood disorders, Libido decreased, Headache, Hot flush, Nausea/ vomiting, Upper abdominal pain, Constipation, Elevated liver enzymes, Hyperhidrosis, Night sweats, Arthralgia, BMD decreased, Vaginal haemorrhage, Pelvic pain, Change in menstrual bleeding pattern, Vulvovaginal dryness, Asthenia. Uncommon (>1/1,000 to <1/100): Libido decreased, Upper abdominal pain, Constipation, Night sweats, BMD decreased, Arthralgia, Vulvovaginal dryness, Changes in menstrual bleeding pattern, Asthenia. Please refer to the Summary of Product Characteristics for a description of selected adverse reactions including mood disorders, elevated liver enzymes, bone mineral density changes and vaginal haemorrhage. Package Quantities & Cost: 28 film- coated tablets, £80. Marketing Authorisation numbers: PLGB 49876/0023, PLGB 49876/0024. Marketing Authorisation holder: Theramex Ireland Limited 3rd Floor, Kilmore House, Park Lane, Spencer Dock, Dublin 1 D01 YE64 Ireland. Legal classification: POM. Job code: YSELTY_HQ-UK_EN_21717_v1. Date of Preparation: March 2025.
| Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to Theramex on [email protected] or Tel: +44 (0)333 0096795 |