INTRODUCTION
Bronchoscopic lung volume reduction (BLVR) with endobronchial valves has established effectiveness in improving lung function in patients with COPD who do not have alpha-1 antitrypsin deficiency (AATD).1,2 However, the effect of BLVR on patients with AATD is less certain. Several small-scale studies have shown a benefit using BLVR in patients with both AATD and COPD.3,4 The authors have previously demonstrated that 6-month BLVR outcomes were similar in patients with COPD, with or without AATD.5 However, there are currently no published studies comparing long-term BLVR outcomes between these two patient groups. This study thus aimed to compare outcomes at 12 months post-BLVR.
METHODS
The authors conducted a retrospective, single-center, matched case-control study involving patients with COPD who underwent BLVR. A thorough chart review was performed for 287 patients who underwent BLVR at the authors’ quaternary care institution between August 2018–April 2024. The chart review revealed 14 patients with severe, homozygous AATD within this group. 14 patients with COPD, but without AATD (nAATD), were selected from the remaining 273 BLVR patients to be matched against the AATD group. Baseline characteristics including demographics, fissure integrity, emphysema destruction score, quantitative computed tomography lobar volume, and baseline pulmonary function testing were recorded. Mean values were compared between groups using an equal variance t-test. Pulmonary function testing data from approximately 12 months following BLVR was then collected. Post-BLVR data was compared to baseline data and analyzed using Wilcoxon signed rank test for statistical significance in each group independently. Changes in several variables, including forced expiratory volume in 1 second (FEV1), FEV1 percent change, residual volume (RV), and total lung capacity (TLC), were compared between the two groups using an equal variance t-test.
RESULTS
Baseline characteristics had statistically significant differences in emphysema destruction score (69.92±7.77 versus 61.64±11.62; p=0.04) and modified Medical Research Council score (3.78±0.43 versus 2.85±0.66; p=0.002). There were no other differences in baseline characteristics. Following BLVR, seven patients (50%) in the AATD group and nine patients (64%) in the nAATD group experienced an increase in FEV1 greater than 15%. The median improvement in FEV1 was 125 mL (interquartile range [IQR]: 0.04–0.24), which equated to 13.94% (IQR: 5.87–30.09), in the AATD group and 155 mL (IQR: -0.07–0.25), which equated to 17.99% (IQR -8.09–29.67), in the nAATD group. Both improvements were statistically significant per Wilcoxon signed-rank test analysis. Statistically significant improvement was also demonstrated in both groups for changes in RV, TLC, and RV/TLC ratio. The improvements in FEV1, FEV1 relative percent change, and TLC were not significantly different between the two groups. However, there was a statistically significant difference between the groups, in terms of RV and RV/TLC, which demonstrated more pronounced improvements in the AATD group. A summary of outcomes is illustrated in Table 1.
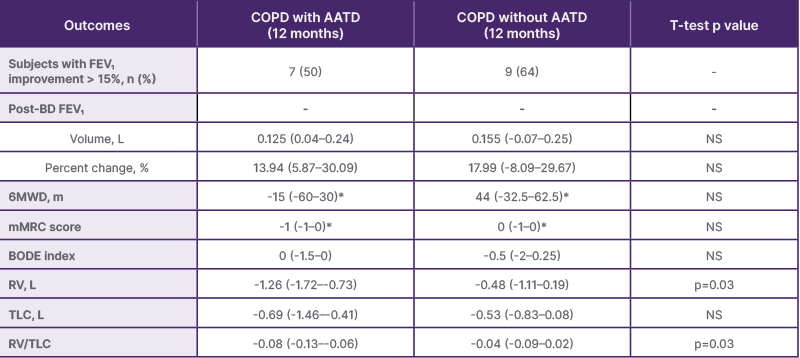
Table 1: Summary of outcomes.
*No statistically significant change from baseline. Values are medians with interquartile ranges. Wilcoxon signed rank test performed for each change in baseline. All values except for those marked by an asterisk are statistically significant changes from baseline. All t-tests described as ‘NS’ have p>0.05 indicating no statistically significant difference between group outcomes.
AATD: alpha-1 antitrypsin deficiency; BODE: BMI, airflow obstruction, dyspnea, exercise capacity; FEV1: forced expiratory volume in 1 second; mMRC: modified Medical Research Council; NS: non-significant; post-BD: post-bronchodilator; RV: residual volume; TLC: total lung capacity; 6MWD: the distance walked in 6 min.
CONCLUSIONS
BLVR may provide significant long-term improvements for patients with both COPD and AATD. These improvements may be similar to those expected for COPD patients without AATD. The lack of airway disease in patients with AATD may allow for more pronounced improvements in certain variables. Further research on a larger scale is warranted to support these findings and investigate the safety profile of this intervention.







