Abstract
Background: Latent tuberculosis testing prior to immunosuppression is recommended by national and international guidelines. However, no structured process currently exists for testing adult patients at tertiary care hospitals in Calgary, Alberta, Canada. Additionally, there is limited data describing interferon-γ release assay, QuantiFERON® (QFT; QIAGEN, Hilden, Germany) among inpatients. In 2021, a QFT order set was implemented for hospitalized patients with the aim to facilitate rapid testing prior to immunosuppression. This study aimed to compare the proportion of in-hospital immunosuppression started prior to QFT collection before and after QFT order set implementation, and to assess variables associated with indeterminate test results.
Methods: The authors performed a retrospective chart review of adult inpatients who underwent QFT at four acute care hospitals from 2020–2022. The Z-test was used to compare the proportion of pre-immunosuppression QFT testing pre- and post- order set implementation. Associations were analyzed using logistic regression.
Results: A total of 639 inpatients had QFT testing. The most common indication for QFT testing was immunosuppression. The proportion of patients who began immunosuppression prior to QFT decreased following order set implementation (54% versus 45%; p=0.0388). Indeterminate QFT results were associated with immunosuppression initiation before QFT (p<0.005). In multivariable analysis, the odds of an indeterminate QFT increased for patients with low lymphocyte counts (adjusted odds ratio [OR]: 3.45; 95% CI: 1.49–7.69) or those who received prednisone ≥50 mg (adjusted OR: 1.84; 95% CI: 1.0–3.39).
Conclusion: QFT should be performed before immunosuppression and, if possible, prior to hospitalization. Despite implementation of a QFT order set, immunosuppression was still frequently started before QFT collection. Further work is needed to identify barriers to early testing and evaluate strategies to optimize screening.
Key Points
1. Latent tuberculosis infection (LTBI) testing prior to immunosuppression is recommended by both national and international guidelines. Many hospitalized patients require urgent immunosuppression, however there is limited published data regarding LTBI testing in this population.2. In this cohort of adult patients hospitalized at four tertiary acute care hospitals, immunosuppression was the most common reason for LTBI testing.
3. Starting immunosuppression before LTBI testing using QuantiFERON® was associated with an increased rate of indeterminate results. Despite this, many patients in the authors’ cohort received high-dose immunosuppression prior to QuantiFERON. More work is required to optimize inpatient LTBI testing.
INTRODUCTION
Latent tuberculosis infection (LTBI) affects up to one quarter of the worlds’ population.1 Although Canada is a low-incidence nation, the majority of incoming migrants originate from endemic areas, and it is estimated that over 1.5 million people carry the infection.2 Patients with LTBI who require immunosuppression are high risk for tuberculosis (TB) reactivation. Both the WHO and Canadian Tuberculosis Standards (CTS; 2022) recommend systematic testing and treatment of patients initiating immunosuppressive drugs associated with high risk for TB reactivation.3,4 Despite these recommendations, there is no structured process in Calgary, Alberta, Canada, to ensure LTBI screening is performed before the initiation of immunosuppression in hospitalized patients.
LTBI testing can be performed with a tuberculin skin test (TST) or an interferon (IFN)-γ release assay (IGRA). QuantiFERON® (QFT) is the IGRA test that is used in Alberta. Locally, there has been a shift to QFT-based testing for inpatients due to several advantages, including single blood draw collection, improved specificity, and more objective results.4 However, there is limited published data describing the results of QFT among inpatients and none, to the authors’ knowledge, in a Canadian setting.5,6
Until 2021, ordering an inpatient QFT in Alberta required approval from a TB physician. This may have delayed testing, and anecdotally, LTBI testing was delayed by days to weeks after immunosuppression initiation. Immunosuppression prior to LTBI testing is associated with an increased rate of indeterminate results, thereby reducing the clinical utility of the test.6-8 In order to streamline the ordering process, a QFT order set was implemented in 2021 for inpatients at all acute care hospitals in Calgary. This allowed providers to order a QFT for patients requiring immunosuppression without TB physician approval, facilitating rapid testing prior to immunosuppression.
The objectives of this study were:
- To compare the proportion of in-hospital immunosuppression started prior to QFT collection before and after QFT order set implementation; and
- To assess patient variables associated with indeterminate test results among hospitalized patients.
METHODS
The authors performed a cross-sectional study using retrospective chart review data from all adult inpatients who underwent a QFT at any of the four acute care hospitals in Calgary, between January 1st–October 31st 2020 (prior to QFT order set implementation), and January 1st–December 31st 2021 (post QFT order set implementation). Calgary, has four publicly funded acute care hospitals for adults, and the general population is able to access any center. Demographics between hospitals were not expected to differ significantly, except based on geographic location. The QFT order set was implemented in November 2020; thus, the period fromNovember 1st–December 31st 2020, was excluded from data collection to allow for roll-out/awareness of the order set. The study cohort was identified from the Alberta Precision Laboratories Public Health database, which performs all QFT testing in Alberta.
Inclusion criteria were: age 18 years or older at the time of QFT collection; acute care inpatient at time of testing; and a valid QFT result (positive, negative, or indeterminate). Electronic charts were manually reviewed, and data was abstracted using a structured form. The first ten charts were reviewed by two reviewers (Julien Ferland, McGill University, Montreal, Canada, and Leila Barss, University of Calgary, Canada) for consistency in data abstraction. For patients with duplicate testing, the result from only the first test was used for analysis.
Demographic variables including age, sex, co-morbid conditions, and home immunosuppression medications were collected. Ethnicity was collected if noted in admission documentation. Details of immunosuppression medication administered in hospital were recorded, including medication type, dose, and the timing of first administration. New immunosuppression was defined as any new immunosuppressive drug started or escalation in the home medication dose, between hospital admission and QFT collection. The indication for immunosuppression and QFT testing was collected by chart review for all patients. The QFT order set allowed providers to indicate if the QFT was for immunosuppression or ‘other (e.g. recent close contact or recent immigration)’. For ‘other’, TB physician approval was required. This information was also collected for all patients who underwent a QFT in the ‘post order set implementation’ period and used to assess the appropriate use of the order set; specifically whether providers were using it to bypass TB physician review when ordering QFTs for indications other than immunosuppression. Lymphocyte count, C-reactive protein (CRP), and albumin results were collected if available within 72 hours of QFT collection. QFT results, including mitogen and nil results, were collected from the Alberta Precision Laboratories Public Health Laboratory database.
QFT testing was done using QuantiFERON-TB® Gold Plus (QFT-Plus; QIAGEN, Hilden, Germany) for all patients. Incubation was performed according to manufacturer’s guidelines, and results were classified as positive, negative, or indeterminate.9 Blood samples were collected by trained phlebotomists for all hospitalized patients, except for patients admitted to the ICU, where either a bedside nurse or phlebotomist collected samples.
The primary outcome of this study was the proportion of patients who began immunosuppressive treatment prior to QFT collection in the pre- versus post-order set periods. Secondary outcomes included the number of patients with indeterminate QFT results and an examined association of five a priori factors for association.
Baseline characteristics among patients who underwent a QFT for immunosuppression screening were compared among the pre- versus post-order set implementation period using Pearson’s chi-square tests. Because the two groups were independent, a two-proportion Z-test was used to assess proportions of pre-immunosuppression QFT testing. To identify variables associated with indeterminate results, a univariate and multivariable binary logistic regression was performed on the entire cohort. The variables tested were age, sex, year of study, hospital site, and factors known to possibly influence the QFT result: timing of immunosuppression, autoimmune disease, prednisone dosage, albumin level, CRP level, and lymphocyte count below the lower limit of normal. All analyses were performed using Stata version 16.1 (StataCorp LP, College Station, Texas, USA).
This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB 22-0365). Patient informed consent was waived due to the retrospective nature of the study.
RESULTS
Patient Characteristics and QuantiFERON Test Results
Between January 1st–October 31st 2020, and January 1st–December 31st 2021, 643 QFT results were reported from hospitalized patients at the four adult acute care hospitals in Calgary. Four results were duplicate tests and were excluded from analysis. Of the 639 patients included in analysis, there were 224 QFT results in the pre-order set implementation period (2020) and 415 QFT results in the post-order set implementation period (2021).
The most common indication for QFT testing was immunosuppression in both periods (85% in pre-order and 83% in post-order; Supplementary Table 1). Characteristics for patients undergoing testing due to immunosuppression are shown in Table 1. There was no significant difference in the proportion of patients taking home immunosuppressive medications prior to hospitalization (26% pre- versus 21% post-order; p=0.16). In the pre-order set period, vasculitis was the most common diagnosis associated with immunosuppression testing (20%), whereas inflammatory bowel disease (25%) was most common in the post-order set period.
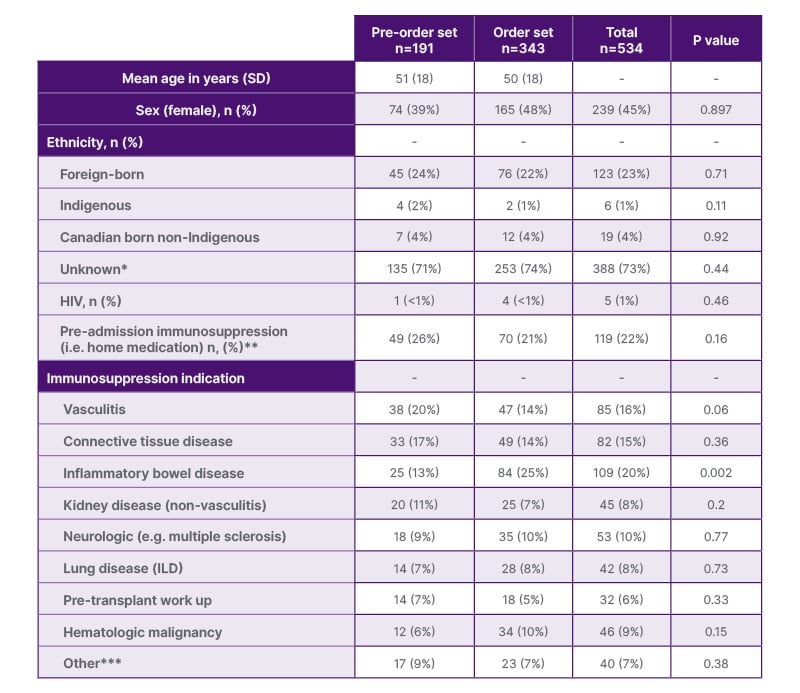
Table 1: Baseline characteristics among population undergoing QuantiFERON® for immunosuppression screening.
*Ethnicity not mentioned in admission documentation
**Defined according to admission date: steroid within 2 weeks; chemotherapy, immunotherapy, or biologic within 6 weeks; rituximab within 6 months
***Hemolytic anemia, myopericarditis, allograft rejection, dermatologic drug reaction, immunotherapy mediated colitis, immune thrombocytopenia, psoriasis, COPD requiring prolonged steroid taper
ILD: interstitial lung disease.
Among the entire cohort of 639 patients, QFT results were positive in 34 (5%), negative in 497 (78%), and indeterminate in 108 (17%). Among the cohort of 534 patients undergoing testing for immunosuppression, QFT results were positive in 23 (4%), negative in 415 (78%), and indeterminate in 96 (18%). There was no significant difference in indeterminate results between pre- versus post-order set periods (21% versus 16%; p=0.18).
Immunosuppression Order Set Implementation
There was a significant decrease in the initiation of inpatient immunosuppression prior to collection of QFT following implementation of the order set (103/191 [54%] versus 153/343 [45%]) in the pre-order set versus the post-order set period, respectively (p=0.0388). Steroids were the most common immunosuppressive medication started prior to QFT collection, administered in 99/151 (52%) patients in the pre-order set period and in 146/343 (43%) of patients in the post-order set period (Supplementary Table 2[/hl]).
The immunosuppression order set was appropriately used in 313/343 (77%) patients. This included cases where QFT testing was due to ‘immunosuppression’ and ‘immunosuppression’ was selected, or QFT was done for an ‘other’ indication and ‘immunosuppression’ was not selected. Incorrect order set use was due to provider selection of ‘immunosuppression’ on the order set for non-immunosuppression testing (e.g. test was performed as part of active TB work up) in 26/343 (6%) of patients, and due to ‘immunosuppression’ not being selected on the order set for immunosuppressive QFT (based on chart review) in 69/343 (17%) of patients.
Indeterminate QuantiFERON Analysis
An indeterminate QFT result was associated with immunosuppression initiation before QFT collection (Chi-square [1; n=639]: 20.55; p<0.005). Results of the univariate and multivariable analyses are presented in [hl]Table 2 and Supplementary Table 3. The multivariable analysis included timing of new immunosuppression prior to QFT, prednisone dosing, autoimmune disease, albumin, CRP, and lymphocyte count. In the multivariable analysis, the odds of an indeterminate QFT were significantly increased if patients had an abnormally low lymphocyte count (adjusted odds ratio [OR]: 3.45; 95% CI: 1.49–7.69) or received daily prednisone dosed at ≥50 mg (adjusted OR: 1.84; 95% CI: 1.0–3.39). Steroids started before QFT in 96% of those receiving immunosuppression, with 56% receiving high-dose steroids. The frequency of other immunosuppressive medications were too low to analyze individually in association with QFT results.
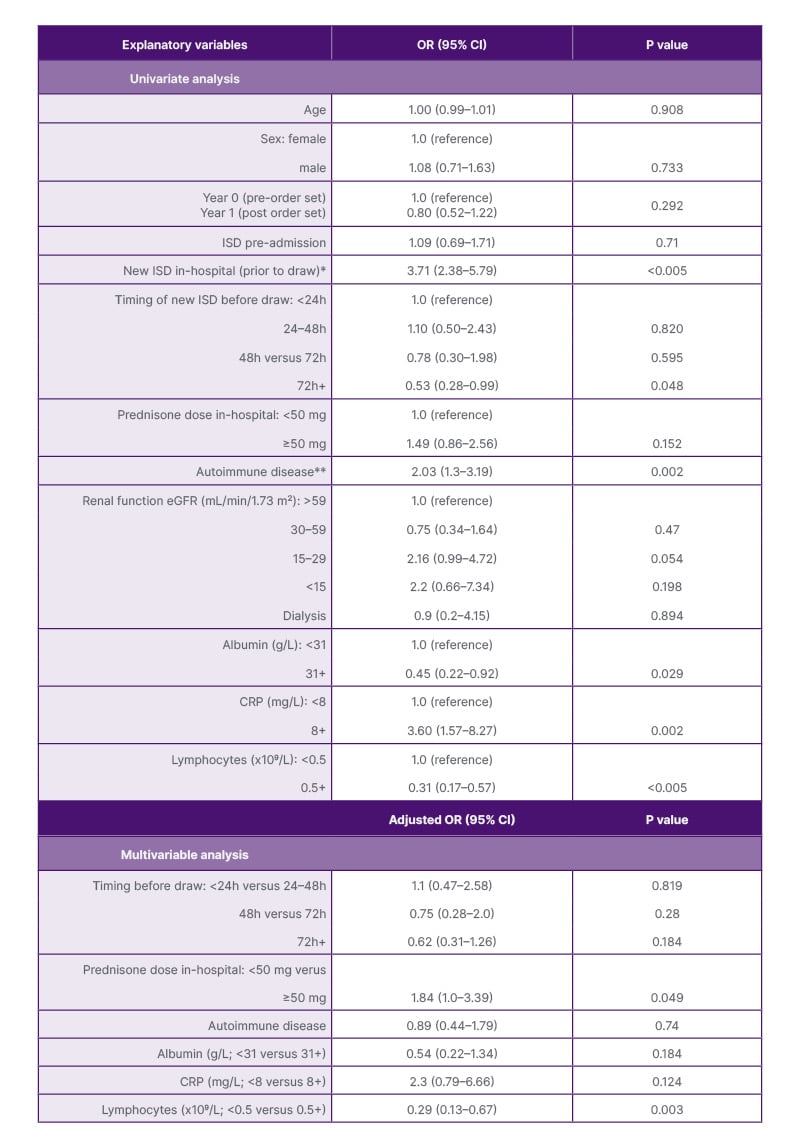
Table 2: Univariate and multivariable logistic regression analyses of factors associated with indeterminate QuantiFERON® result.
*Defined as either new start of immunosuppression and/or increased home dose administered before QFT collection.
There was no difference in the rate of IND results among patients started on new immunosuppression versus
those who had escalation of their home immunosuppression (Supplementary Table 3).
**Vasculitis, neurologic, inflammatory bowel disease, or connective tissue disease
CRP: C-reactive protein; eGFR: estimated glomerular filtration rate; ISD: immunosuppressive drug; OR: odds ratio.
Elevated CRP, lower lymphocyte counts, and the use of immunosuppression in hospital was associated with lower IFN-γ responses (p<0.005, p=0.008, and p<0.005, respectively). Indeterminate results were due to a low mitogen response in all except one patient.
DISCUSSION
Inpatient latent TB screening is often required urgently to prevent iatrogenic TB reactivation in the face of impending immunosuppression for acute illness. To the authors’ knowledge, this is the largest retrospective study assessing indications for QFT use among hospitalized patients and the relationship between test timing and inpatient immunosuppression medication. Among the authors’ cohort of hospitalized adult patients in Calgary, over 80% of QFTs performed in hospital were for the indication of immunosuppression medication initiation/escalation. Immunosuppression was frequently started before QFT collection, despite the potential adverse impact on test sensitivity. Several studies have determined that immunosuppressive medications can result in higher rates of indeterminate and even falsely negative results.10-12 Similarly, this study found that immunosuppression initiated in hospital prior to QFT collection was associated with an indeterminate result. In the multivariable analysis, the timing of initiation relative to QFT (e.g. started <24 hours prior to the test versus started >72 hours prior to the test) was not associated with an indeterminate result, but dosing of steroids (>50 mg prednisone equivalent) did significantly increase the odds of an indeterminate result (adjusted OR: 1.84; 95% CI: 1.0–3.39).
The implementation of an electronic QFT order set may have reduced the proportion of patients who had delayed QFT testing. The electronic order set streamlined ordering by reducing the time needed to speak with a TB physician prior to ordering every test, and also reduced the need for laboratory staff to confirm the test was approved. However, there was still a large proportion of patients started on immunosuppression prior to QFT collection (reduced from 54% to 45%). The order set was also used incorrectly in almost a quarter of patients, indicating that a lack of clinician education/awareness around the ordering process may be a contributor to suboptimal screening. We found that 11% of QFTs were ordered as part of the work-up for active TB, which is generally not recommended due to inadequate specificity and sensitivity (possibility of anergic response in active disease).13 Bouton et al.14 showed that almost two-thirds of QFTs done in their regional health network were ordered for the purposes of ruling out active TB, demonstrating that this may be an ongoing knowledge gap. Other strategies to optimize screening practices for TB infection, particularly before immunosuppression is initiated, are needed considering the rising frequency of immunosuppressive conditions and medication use among the general population.15 An accurate and timely TB infection diagnosis is essential for providing effective TB preventative therapy among immunosuppressed patients. Indeterminate results may delay initiation of immunosuppressive treatment and/or lead to inappropriate LTBI treatment choices, due to lack of an accurate diagnostic test. The importance of this cannot be understated, as preventative therapy has been shown to be up to 90% effective in reducing the incidence of reactivation TB, which carries significant morbidity and mortality.16
Interestingly, the indeterminate QFT frequency of 17% among the author’s entire cohort was much higher than reported among both immunocompetent (1.9%) and immunocompromised (5.7%) populations in a recent meta-analysis.8 While immunosuppressive medications in the author’s cohort was a factor, the indeterminate rate was still high (10%) among inpatients with no immunosuppression at the time of testing. Three previous studies also reported a high frequency of indeterminate results (20–27%) among hospitalized patients.5,6,14 One study found that being an inpatient had an OR of 8.6, compared to outpatients.6 Another study found that inpatients were 11 times more likely to have an indeterminate result compared to outpatients.14 A few of these studies surmised that this phenomenon could be related to collection factors (residents/bedside nurses versus trained phlebotomists). However, in the author’s cohort, all testing, except patients from the ICU, was performed by trained phlebotomists. Therefore, there are likely additional host factors associated with acute illness and hospitalization that contribute to the high indeterminate rate among the author’s cohort.17
One such host characteristic is lymphocyte count, as a low lymphocyte count was associated with an indeterminate result in the author’s study. Insufficient lymphocytes or an inability of the patient’s lymphocytes to generate IFN-γ can result in an indeterminate assay result, due to a low IFN-γ response to mitogen.18 Previous studies have also reported an association between low albumin and lymphocytes with increased frequency of indeterminate QFT results.5,19 Both can be related to the severity of illness among acutely unwell patients, suggesting that disease severity and consequent immune function may affect QFT results.17,20
A limitation to this study includes the absence of standardized LTBI testing criteria for hospitalized patients (physician discretion of whom to test and when). Disease severity was not assessed, it is possible that more severe inflammatory disease may be associated with higher rates of indeterminate QFT results. These patients may be more likely to start on early immunosuppression (prior to QFT collection), which could impact the results. Finally, due to the retrospective nature of this study, the authors were not able to collect data on provider awareness of the QFT order set and the impact this may have had on QFT ordering patterns. An important strength of this study is that a detailed chart review was undertaken to determine accurate indications for QFT orders and other patient characteristics, rather than reliance on administrative data like International Classification of Diseases (ICD) codes, to facilitate an exploratory analysis of risk factors for an indeterminate result.
CONCLUSION
Initiation of immunosuppression medication was the most common reason for QFT use among the cohort of hospitalized patients. QFT should be performed before immunosuppression initiation, and if possible, prior to hospitalization to obtain an accurate TB infection status and facilitate appropriate TB preventative therapy among this high-risk population. Implementation of an inpatient QFT order set reduced delayed testing in this cohort. However, immunosuppression was still started before QFT collection in a large proportion of patients. Further work is needed to identify barriers to early inpatient testing and evaluate strategies to optimize screening among hospitalized patients.







