Abstract
Asthma remains a leading cause of preventable morbidity and healthcare use worldwide, despite the availability of effective preventative inhaled therapies. A major contributor to this persistent gap between expected and observed outcomes is suboptimal adherence to inhaler treatment and failure to act appropriately when symptom control is lost. Inhalers with integrated or attached electronic components that record medication use and related information are promising technologies to address these challenges. Evidence from adult and paediatric studies indicates that smart inhalers can improve adherence. They may also improve self-management; for example, through integration with a digital personalised asthma action plan. Advances in digital connectivity and data integration also open opportunities for predicting exacerbations, as well as optimising and tailoring treatment decisions. Despite their promise and increasing evidence, there has not been a widespread adoption of this technology. Barriers to adoption include patient acceptability, data privacy, interoperability of digital platforms, clinician workload, and environmental sustainability related to electronic waste. While economic modelling and early implementation studies suggest potential cost-effectiveness, the key question of ‘who pays’ remains unresolved. Overall, smart inhalers offer a promising avenue to reduce preventable harm in asthma, but their integration into guidelines and practice requires ongoing coordinated progress in technological design, healthcare system readiness, and sustainability planning.
Key Points
1. Asthma is highly prevalent, and effective treatments are outlined in established guidelines. However, poor symptom control and exacerbations remain common. Key drivers of adverse outcomes include low concordance with preventer therapy and lack of timely action when control deteriorates.2. Smart inhalers are those that include electronics that can provide reminders, record usage, and assess technique. This provides physicians and users objective evidence around concordance. Linking information to other data streams has the potential to predict serious events and allow earlier intervention.
3. While the use of smart inhalers has shown potential for several years, implementation has been limited. This review highlights uncertainties that persist regarding justification of their additional cost, data and management issues, and their environmental impact.
INTRODUCTION
Asthma is a highly prevalent condition, affecting over 250 million people worldwide.1 Despite widespread recognition and the availability of effective therapies, asthma continues to cause significant global harm. Each year, hundreds of thousands of deaths are attributed to asthma, alongside millions of instances of urgent healthcare use and substantial individual morbidity from the disease and oral corticosteroid treatment.1 This harm occurs in the context of decades of availability of effective preventative therapy, including in countries where such medications are readily accessible and are broadly affordable.2 The use of these therapies is supported by international guidelines describing appropriate deployment and monitoring, and cataloguing the extensive available supporting study data. The persistent gap between the expected positive treatment outcomes and observed reality is multifactorial, but not using preventative treatment as prescribed is a key driver. It has also been repeatedly shown that most people with asthma and their general practitioners accept regular symptoms,3 and often do not recognise poor control as a prompt to act to avoid more serious deterioration.4
This review will consider the two centrally important issues of inhaler adherence and early appropriate intervention during exacerbations, before giving examples of the research evidence describing the potential for smart inhalers to begin to address these problems. Given the encouraging data supporting their use, it is notable that the widespread adoption of smart inhalers has not occurred. The barriers to incorporating such devices into the standard ecosystem of asthma care will thus be outlined.
WHAT ARE SMART INHALERS?
Inhalers may be described as ‘smart’ if they include embedded or attached electronic devices that can record drug dosing, timing, and, in some cases, the appropriateness of the inhalation pattern. Since their development over 30 years ago,5 there have been advances both in these devices and the technological infrastructure to support them.6 Today, smart inhalers are capable of unobtrusively recording relevant information and wirelessly connecting to a smartphone to allow data to be analysed and presented back to the user through an app, before being rapidly transferred to their clinician. Commercially available smart inhalers have varying capabilities, such as recording reliever and/or preventer inhaler usage, location, inhaler technique, and audiovisual reminders. Smart devices are available for common device types, including pressurised metered-dose inhalers, soft mist inhalers, various dry powder device types, or as attachable sensors to existing inhalers. This opens up the possibility for meaningful patient interaction, such as medication use reminders and inhaler technique feedback. It also gives clinicians a better insight into how the patient is using preventative inhaled therapy before considering a step-up to (usually) more costly and complex add-on therapies.7 Figure 1 gives examples of initial smart inhalers that were largely a casing for a pressurised metered-dose inhaler cannister, and newer devices that clip or stick on to standard inhalers.
Figure 1: Examples of smart inhalers illustrating older devices that encompass pressurised metered-dose inhaler cannisters (Smartinhaler Tracker) and newer devices that attach to standard devices (Hailie® Smartinhaler®).
Hailie® Smartinhaler®: Adherium, Melbourne, Australia; Smartinhaler Tracker: Adherium (formerly Nexus6), Auckland, New Zealand.
HOW IS ADHERENCE AND CONCORDANCE MEASURED?
Adherence is broadly defined by the WHO as “the extent to which a person’s behaviour corresponds with agreed recommendations from a health care provider.”8 Suboptimal adherence to therapy has long been a barrier to optimised care, and is strongly correlated with high morbidity, mortality, healthcare resource utilisation, and reduced quality of life.9 Within the EU, medication non-adherence has generally been associated with almost 200,000 deaths annually.10 It is important to emphasise that adherence is not a static characteristic; shifts in patient perspectives, behaviours, experiences, and settings can influence how medications are taken. For example, there may be greater medication use during high-risk seasons, in response to worsening symptoms, or following new information (accurate or otherwise). In this context, although there are some associations identified, there is no reliable panel of predictive factors for determining suboptimal adherence.11 Patterns of medication use can be affected by age, gender, comorbidities, personal behaviours, symptom perception, and the individual’s knowledge of their underlying disease. External factors such as socioeconomic status, access to healthcare, disease burden, and the complexity of prescribed therapy also play significant roles.
The term ‘concordance’ is also used in discussions of treatment behaviour and refers to a more patient-centred approach to care.12 Unlike adherence, which focuses on whether medications are taken exactly as directed by the clinician, concordance emphasises a collaborative process where healthcare providers and patients agree on treatment decisions. Using the guiding principle that the best inhaler is the one the patient takes (and takes correctly), concordance can be improved by unveiling potential barriers to inhaler use and using shared decision-making during the prescribing process.
Despite its clinical relevance, concordance is inherently difficult to quantify and is rarely monitored in routine practice or research settings. Instead, adherence is measured; this has its recognised limitations, as it uses methods such as self-reporting, dose counting, and medication possession ratios. To improve consistency across clinical research, the Ascertaining Barriers to Compliance taxonomy initiative was created, providing standardised terminology around adherence patterns and ‘real-world’ medication use.13 Some common adherence patterns are demonstrated in Figure 2 (a theoretical one-puff, twice-daily dosing regimen). Adherence patterns include optimal adherence, with each dose being taken using the correct technique every day, and primary non-adherence, where a patient fails to initiate treatment, such as by not filling their prescription. Estimates of primary non-adherence vary between study designs and settings, but appear to be in the range of 10–30%.14-16 Non-persistence, where treatment is initiated correctly but ceased by patients without medical consultation, and unintentional non-adherence, where patients struggle with inhaler technique or lack the resources to maintain regular use, are also observed adherence patterns.17,18 Poor inhaler technique is common, and even apparently minor errors are associated with worse outcomes. Some people also show non-conformance, which is characterised by a self-adjustment of their regimen, e.g., taking more or fewer doses than recommended.
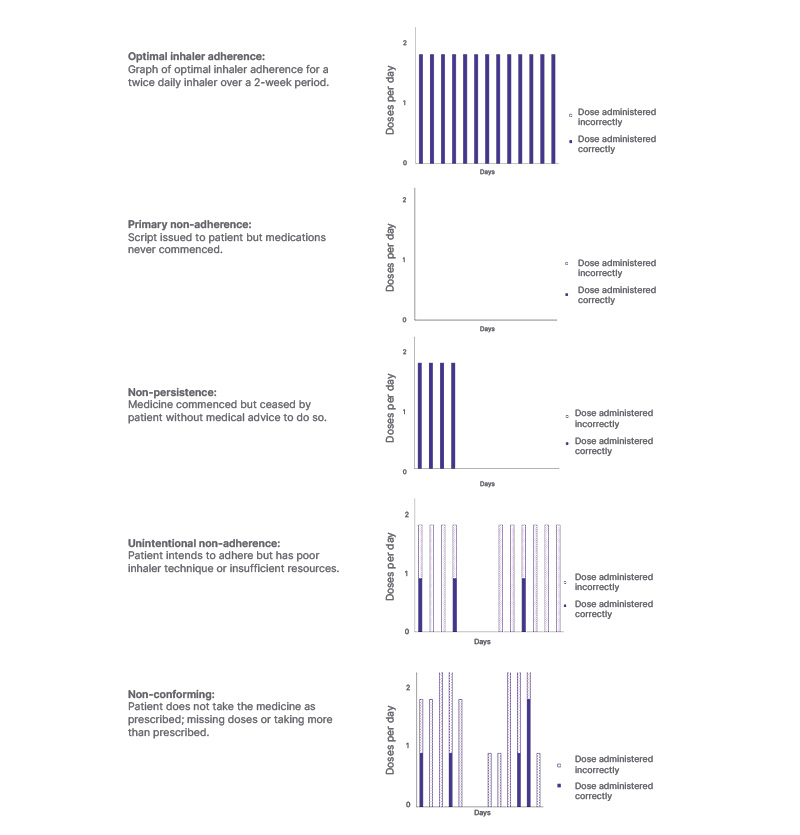
Figure 2: Patterns of inhaler use demonstrating the types of suboptimal adherence.
These are representative graphics to illustrate concepts, not based on individual patients’ data. The first graph represents optimal adherence, the second graph illustrates primary non-adherence, the third graph depicts non- persistence, the fourth graph reflects unintentional non-adherence, and the fifth graph shows non-conformance.
Identifying and understanding adherence patterns in busy clinical settings can be challenging. Validated questionnaires, such as the Test of Adherence to Inhalers (TAI), can be completed prior to an appointment, and allow patients to self-report their inhaler use behaviours.19 Although helpful, they do not replace objective measures, and in the authors’ experience, over-reporting of medicine use is still common.
DO SMART INHALERS IMPROVE CONCORDANCE?
Digital technologies can support improved adherence to all aspects of respiratory care, including medication use.20 Smart inhalers equipped with or initiating audiovisual reminder feedback (AVRF) have demonstrated an increase in medicine adherence and provide healthcare professionals with valuable insights into the use of the smart inhaler, be that preventer, reliever, or both. For example, in 2007,21 a randomised, open-label, controlled trial using the Smartinhaler device investigated the impact of AVRF from a smart inhaler on treatment adherence. There was a notable improvement in adherence in the intervention group, but the difficulty of studying this real-world issue in a trial was also highlighted: adherence was high, at over 70% in both arms. Further randomised studies in adults (using the SmartTrack system by Nexus6 [now Adherium, Melbourne, Australia]; and Propeller Health clip [Propeller Health, acquired by ResMed, San Diego, California, USA] on devices) have supported these findings.22,23
Positive outcomes with smart inhalers were also seen in a paediatric asthma cohort following their emergency department presentations.24 Improved adherence to preventer therapy was seen in children who received smart inhalers with enabled AVRF (84%) compared to those who received a smart inhaler without AVRF (30%). Further studies in paediatric respiratory clinics demonstrated that children who also received feedback from their clinicians had higher adherence rates than those without feedback.25,26
Following these insights, a key question remained: is smart inhaler technology better than intensive patient education alone? This was explored by Sulaiman et al.,27 who investigated the benefits of INCA™ smart inhalers, Vitalograph, Ennis, Ireland, on adherence and inhaler technique in comparison to intensive education. Adults with severe uncontrolled asthma were given smart inhaler preventers with repeated inhaler training, adherence education, and disease management, with monthly re-education. The intervention group received the same education but enhanced with (bio)feedback-guided training based on their smart inhaler data, with inhaler sensors able to detect critical inhaler errors in addition to missed doses. The addition of (bio)feedback using smart inhaler data significantly improved adherence (73% versus 63%; p≤0.01) and provided valuable information to the clinician on whether adherence was a significant factor regarding poorly controlled asthma for individual patients. Although smart inhalers, their various sensors and capabilities, accessible smartphone apps, digital platform linkage to clinicians, dosing reminders, (bio)feedback mechanisms, and opportunities for education are potentially valuable to patients who are engaged in their healthcare, they are unlikely to make significant behaviour changes in people who are disengaged.
SMART INHALERS AS A TOOL FOR MONITORING AND EDUCATION
In recent years, there has been an increase in the uptake of anti-inflammatory reliever therapy and maintenance and reliever therapy regimens, which are the preferred step one and step two treatments for asthma.28 Smart inhalers can be integrated into inhaled corticosteroid (ICS)-formoterol regimens; rather than the clinician needing to decide between monitoring reliever use or preventer use, both could potentially be assessed with one device, provided only one inhaler is in use, which is unlikely in the clinical setting.29
Monitoring preventer use with a smart inhaler can potentially provide insights into the barriers to optimal adherence and facilitate more positive health behaviours. Simple reminders and documentation of usage are helpful to both the clinician and the person with asthma, but smart devices can assist in other ways. Monitoring flow rates can assess inhaler technique, allowing documentation of whether the inhaler was used, and used properly.
Information on reliever use can also be combined with symptom tracking to more reliably allow individuals to follow their personalised asthma action plans (PAAP), and to alert their prescribing clinician when control is being lost. Patients with daily symptoms from uncontrolled asthma are at higher risk of exacerbations; however, poor insight and disease knowledge remain common barriers to actioning PAAPs early. Smart inhalers provide an opportunity to enhance the effectiveness of PAAPs, especially if linked to educational materials and providing a record of the time spent in each PAAP zone.
The wider potential benefits of smart inhalers can be appreciated if considered through the lens of the National Institute for Health and Care Research (NIHR)’s Practical systematic Review of Self-Management Support (PRISMS) components relevant to successful self-management.30 Objective inhaler use data linked to other electronic support materials and medical records can support effective self-management, peer support, and appropriate seeking of healthcare professional input (Figure 3).
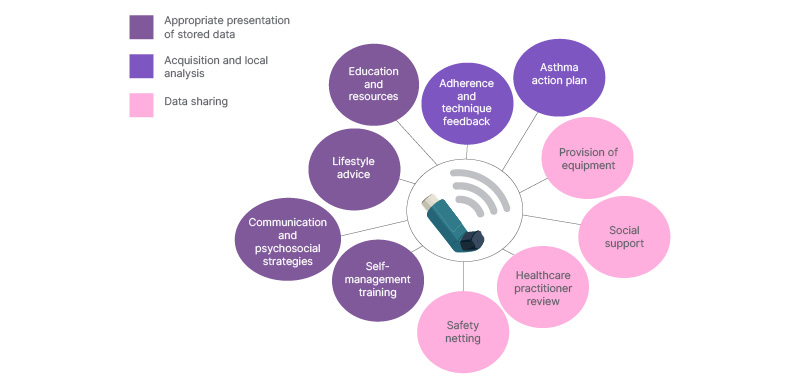
Figure 3: Connected inhaler devices can enhance self-management and personalised care across all categories suggested by the National Institute for Health and Care Research review.30
CAN SMART INHALERS PREDICT ASTHMA EXACERBATIONS?
Lugogo et al.31 demonstrated that smart inhalers attached to relievers (ProAir Digihaler, Teva Pharmaceuticals, Tel Aviv, Israel [discontinued]) can help predict the risk of asthma exacerbation up to 5 days prior to an attack using a machine learning model. The strongest predictor of impending exacerbation was unsurprisingly the mean number of daily reliever inhalations; however, peak inspiratory flow, duration and volume of inspiration, and time to peak inspiratory flow were all important variables. The information gained from smart inhalers may allow for a migration from reactive management of asthma exacerbations towards a proactive prevention approach.
Data from smart inhalers can guide treatment decisions made by healthcare practitioners. Differentiating difficult-to-treat asthma that readily responds to ICS from asthma that is refractory to ICS remains a clinical challenge, and is an important treatment decision in proceeding to long-term biologic therapy. Fractional exhaled nitric oxide (FeNO) is an ICS-responsive biomarker and a useful phenotyping test. FeNO suppression testing can be used to discern if asthma is responsive or refractory to standard ICS/long-acting β-agonist therapy, and hence guide therapeutic decisions.7 Aligning smart inhaler data with FeNO, spirometry, and symptoms can effectively identify the population with difficult-to-treat asthma who can respond well to ICS-based regimens and those who need to progress to biologics.
OPTING IN: SMART INHALERS FOR PERSONALISED MEDICINE
It is exciting to consider the possibilities surrounding smart inhalers in the contribution to personalised management of airway disease. This is especially the case when considering additional linked technologies such as Global Positioning System location information, which could help to identify particular triggers or warn if there is a general increase in reliever use in a local area due to a viral outbreak. However, the first step of personalisation is to decide whether a smart inhaler is right for the individual at all. Constant monitoring of inhaler use, timing, technique, and location may raise concerns around privacy and data protection. Receiving alarms and notifications can feel intrusive, as many people already feel that their step-counting smart watches are. For example, a study assessing the effect of smart inhaler use with feedback in children and adolescents showed that the intervention group had higher rates of broken devices (50% versus 19%), or devices broken beyond repair (37% versus 5%), compared to a group with the same device but no feedback.26
Integrating smart inhalers into other input streams, PAAPs and tailored education may be overwhelming or burdensome for some patients. For example, the iPREDICT trial was a monitoring study involving home spirometry, monitors, smart inhalers (Adherium Smart Touch, Adherium, Melbourne, Australia; and/or the ProAir Digihaler), and phone apps.32 Of the 82% of participants who completed the training required, 39% were withdrawn within the first 4 weeks due to lack of recorded data or response.
Strong physician recommendations to engage in smart inhaler monitoring can also feel coercive, potentially straining patient-clinician rapport. Research published in 201733 investigated the attitudes towards smart inhaler monitoring in adolescents with asthma, a population recognised to be at risk for poor adherence. Although overall positive attitudes towards smart inhaler monitoring were noted, the primary motivator for using the device was to dispel disbelief, through proof of adherence, independence, and responsibility, in their guardians and medical team.
The potential for smart inhaler use is clear, but a blanket deployment appears unwise. Targeted or tailored use of smart systems may prove a more productive and efficient use of the resources required.
CURRENT BARRIERS TO IMPLEMENTING SMART INHALERS
Although smart inhalers have been available since the 1980s, they are not commonly used in clinical practice.5 Indeed, the first smart inhaler approved for use by the FDA (the ProAir Digihaler) was discontinued in 2024, only 5 years later.34 In the preceding section, the acceptability of such monitoring to individuals was highlighted. However, to deliver solutions for the majority who would accept some form of smart inhaler, several major barriers to a widespread roll out have not been fully overcome. Key considerations are highlighted in Figure 4.
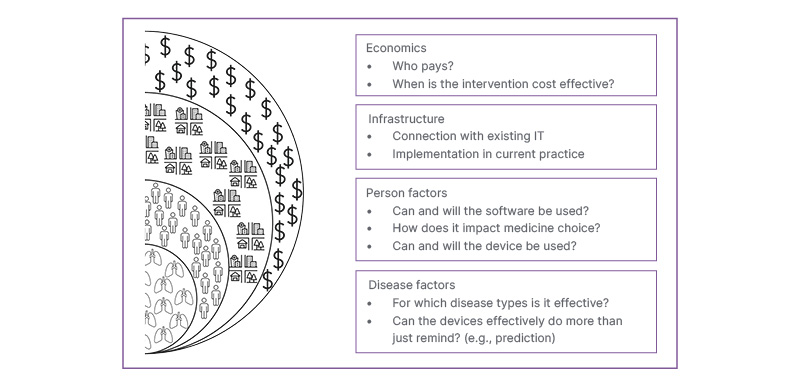
Figure 4: Graphic to illustrate some key barriers that impede the adoption of connected smart inhalers.
As with many medical technologies, some companies have adopted a ‘solution first’ approach and have not actively engaged with patient or clinician groups to produce a product with maximum usability and benefit.35 More recently, projects such as MyAirCoach in the EU and the DIGITAL TEAM RCT in Australia have brought a welcome focus on the development of these technologies to users. However, co-design does not simply include those who hold a physical device.
A key limitation of many technologies and platforms is their lack of interoperability that manifests in three important ways. Firstly, it is unworkable for people with respiratory diseases to download and use different apps for different devices. This is not to imply that they are incapable of using multiple apps simultaneously, but rather that the added complexity reduces the likelihood of meaningful engagement in a real-life setting. The increased complexity introduced by managing workflows including multiple pieces of software also contributes to clinician burnout.36 Secondly, prescribers should not feel constrained in preferred treatment choice by the consideration of which devices’ software is compatible with which medications. Thirdly, many healthcare institutions (particularly public hospitals) have outdated IT infrastructure, run older versions of software and operating systems, and have restrictive security measures; these currently limit the use of a wide variety of diagnostic and monitoring devices across all specialities.37
The increasing use of Health Level Seven (HL7) Fast Healthcare Interoperability Resources standards goes some way towards addressing these issues,38 and towards making data more Findable, Accessible, Interoperable, and Reusable,39 but much work remains.
It is crucial to consider the economics of any healthcare intervention, with devices estimated to cost no less than 100 GBP per year per person, and costs in the intervention arms of studies therefore being higher.40,41 The cost to train staff in newer technologies is a also important; it is not always incorporated into models, but is highlighted as a need by physicians.42 There is a paucity of evidence on the cost-effectiveness of smart inhalers, but the available research appears to be supportive.31,43 This may be expected given the high costs of preventable asthma exacerbations, and of the add-on therapies used for uncontrolled asthma. More practically, in many countries it is not clear who should bear the cost of these connected devices. Manufacturers are understandably unwilling to absorb the costs, most state healthcare systems and insurers are not yet convinced of the benefits of the additional outlay, and devices are usually not directly available to the public. Continuous positive airway pressure devices for sleep apnoea are, in some way, analogous personal connected devices in healthcare: the evidence for their use is more robust, and they are readily available to consumers. However, their cost is not directly covered by Medicare in Australia unless deemed essential to life.44 Should the devices become more widely available to the public, those at higher risk of serious adverse outcomes, such as the socially disadvantaged and elderly people, may not be able to cover these further, out-of-pocket expenses.45 It is perhaps only when the key question of ‘who pays?’ is answered, that we can move forward with appropriately comprehensive economic modelling.
A further important financial consideration is the long delay between the production and approval of medical interventions.46 Developers would have to invest huge sums of money to keep new technologies up to date over the usual approval timescales for medicines,47 and it would seem likely that systems linked to advice, such as an escalation on a PAAP, would require approvals that take years.
In addition to the reservations of users outlined above, clinicians may not wish to be exposed to this additional data. Excess data load is an increasing concern for physicians dealing with electronic health records.48 In areas of medicine where remote monitoring has been introduced, such as with home non-invasive ventilation devices, there also remains a degree of uncertainty as to the extent of responsibility of the prescribing healthcare professional, and to the effectiveness of intensive monitoring.49 More research is required on how to effectively manage and respond to the huge amounts of data arising from connected smart inhaler systems across millions of people with asthma.
SUSTAINABILITY CONSIDERATIONS FOR IMPLEMENTATION
Climate change is having an increasing impact on respiratory health.50 However, the inhaled medicines used to treat the most common conditions are also a source of potent greenhouse gases and single use plastics.51,52 These issues are worsened by inhalers commonly being discarded after partial use, and rarely being either incinerated or recycled to minimise their long-term impact.53 Smart inhalers evidently increase electronic waste, adding a further challenge if incorporated into the billions of devices sold annually. The current transition to new propellants in metered dose inhalers is expensive and complex, and only partially addresses the issues that currently exist.54 To avoid a similar retroactive effort, a great deal of planning and a new infrastructure of recycling is required before widespread adoption of smart devices can reasonably be considered as part of an overall strategy to improve the environmental impact of asthma care.55
SUMMARY
Preventable harm from asthma is common. Key drivers for these adverse outcomes are low adherence to preventative inhaled therapies and failure to respond in an appropriate or timely manner to deterioration in control. Smart inhalers offer an opportunity to remind people to take regular doses, to educate and monitor correct technique, and to tailor support to reduce the frequency of and harm from exacerbations.
Despite high quality supportive research, there has not been a widespread adoption of smart inhalers due to considerations around the acceptability and practical issues with the deployment of technologies. Although progress is being made on these fronts, uncertainties around bearing their cost and in relation to their environmental impact persist. Further work involving multiple stakeholders is required before smart inhalers find their place in guidelines that the supporting evidence suggests they warrant.







