Abstract
Pneumocystis jirovecii pneumonia (PCP) is an opportunistic fungal infection that affects patients who are immunocompromised, which commonly includes people with HIV, haematological malignancies, a history of organ transplantation, and patients on long-term immunosuppressive therapies. England, as well as other countries, has seen a steady increase in the incidence of PCP. The authors’ study aimed to understand which medical conditions were predominant in their patient population diagnosed with PCP. The authors conducted a retrospective and observational study in adult patients with a diagnosis of PCP from January 2019–December 2023, a 5-year period, in a District General Hospital in the UK. The results showed that the majority (55%) of patients diagnosed with PCP did not have any malignancy, and that these patients were mainly individuals with rheumatological or autoimmune disease. Among patients with a prior diagnosis of neoplasia, 71% and 29% had solid organ tumours and haematological malignancies, respectively. The in-hospital fatality rate was 27% among all patients with PCP. There is a rising incidence of PCP among patients on long-term immunomodulating drugs, such as people with autoimmune disorders. A high index of suspicion is required for early disease identification and treatment to reduce mortality.
Key Points
1. There is a rising trend of Pneumocystis jirovecii pneumonia (PCP) infection among patients without HIV with or without cancer.2. Patients with rheumatological and autoimmune diseases are increasingly at risk of PCP.
3. PCP prophylaxis should be considered in high-risk patients on long-term immunomodulating therapies.
INTRODUCTION
Pneumocystis jirovecii pneumonia (PCP) is an opportunistic fungal infection commonly affecting immunocompromised patients, most notably those with HIV, haematological malignancies, a history of organ transplantation, and chronic immunosuppression.1 PCP is caused by the fungus P. jirovecii, previously Pneumocystis carinii, a commensal organism of the respiratory tract.1
In England, the annual incidence of PCP has risen from 2.2 admission episodes per 100,000 population in 2012/2013 to 3.9 admission episodes per 100,000 population in 2021/2022.1 A rising incidence of PCP has also been noted in a German study by Kolbrink B et al.,2 who reported an increase in PCP incidence from 2.3 per 100,000 in 2014 to 2.6 per 100,000 in 2019.
Historically, PCP was predominantly associated with patients with HIV/AIDS. However, with the widespread use of PCP prophylaxis and highly active antiretroviral therapy, the incidence and mortality of PCP in patients with HIV have plummeted.3 Therefore, this rising trend in PCP incidence is related to the increase in the size of the at-risk non-HIV population, a composite of an increasing ageing population, rising prevalence of autoimmune diseases and malignancies, and increased use of immunosuppressive therapies such as B cell-depleting drugs.1 For context, in 2022, Scott et al.4 reported a 42.5% increase in the estimated prevalence of rheumatoid arthritis in England between 2004–2020. This rising trend led to increased exposure to biologics and disease-modifying therapies that, in turn, led to long-term immunosuppression, increasing risks of opportunistic infections such as PCP.5
In a meta-analysis of risk factors for mortality in patients with PCP who are HIV negative, it was noted that haematological malignancies accounted for the majority (29.1%) of non-HIV PCP cases, followed by autoimmune diseases (20.1%; notably systemic lupus erythematosus and rheumatoid arthritis), organ or bone marrow transplantation (14.0%), and solid tumours (6.0%).6 Additionally, most of the study population had a history of receiving immunosuppressive therapies (e.g., steroids or steroids in combination with chemotherapy).6 PCP has high fatality, with a reported mortality rate of 30.6–84.2% in patients without HIV.6-8 Given the rising trend of PCP among patients with autoimmune and rheumatological diseases, the European Alliance of Associations for Rheumatology (EULAR) recommends prophylaxis against PCP in patients treated with daily doses of 15–30 mg of prednisolone or equivalent for 2–4 weeks.9
In this study, the authors examined the characteristics of patients diagnosed with PCP over a 5-year period in their hospital. This study aimed to reaffirm the known disease risk factors for PCP.
METHODS
The authors conducted a retrospective, observational, and descriptive study in adult patients with a diagnosis of PCP between January 2019–December 2023, a 5-year period, in a District General Hospital in the UK. Electronic clinical notes for patients with a diagnosis of PCP were retrieved using International Classification of Diseases, 10th Revision (ICD-10) code B59.
The authors defined a PCP case as a patient diagnosed and treated for PCP based on either clinical manifestations, imaging features or microbiological confirmation, or a clinico-pathological combination.
A descriptive analysis of the variables under study was performed. Two groups were established for comparison: patients with cancer and patients without cancer. The patients with cancer were further grouped into haematological malignancy or non-haematological malignancy. Analysis was performed using Stata 19 statistical software (StataCorp, College Station, Texas, USA).
The study was approved by the Clinical Audit and Research Department of the hospital, and it was exempt from the need for informed consent as it was a retrospective study that did not involve explicit reporting of data for individual patients.
RESULTS
A total of 33 patients were diagnosed with PCP over the 5-year period. Most patients with PCP were men (55%), and the mean age was 62.85 years (SD: 13.80). There was a statistically significant difference between the mean age of patients who died in hospital compared to survivors (two-sample student t-test, one-tailed p=0.03; Table 1). Shortness of breath was the most prevalent symptom (67%), followed by fever (33%).
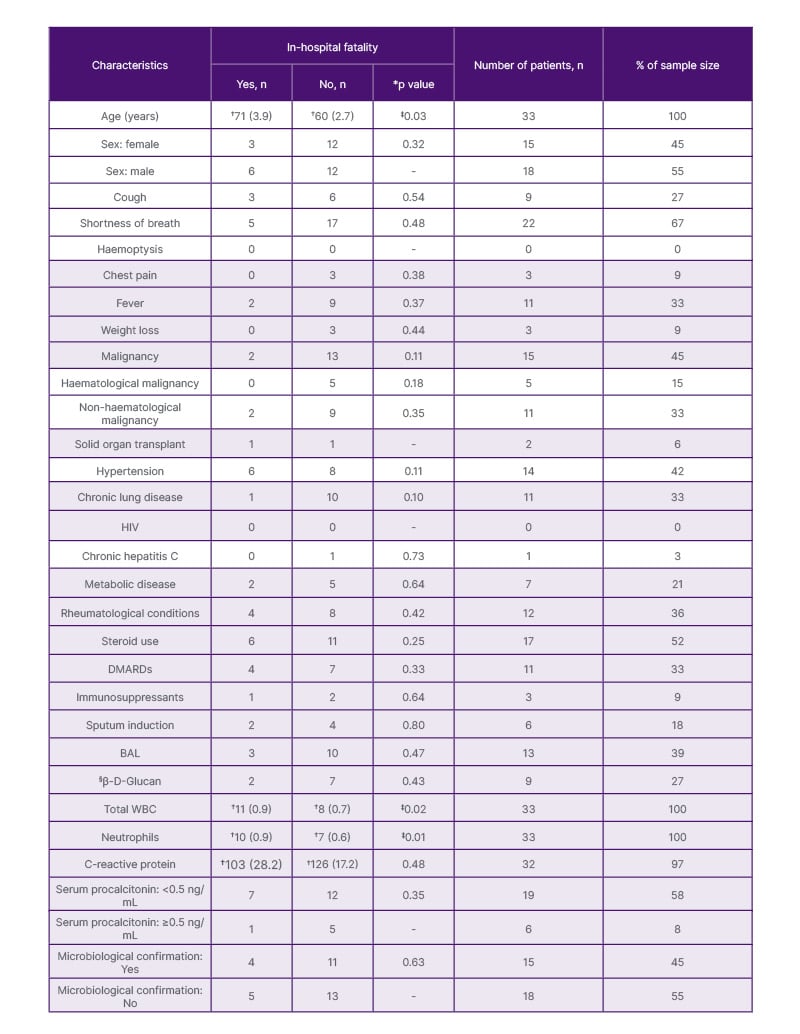
Table 1: Baseline characteristics and in-hospital mortality.
*p value at 5% significance level was derived from a one-tailed Fisher exact test, except for age, total WBC, neutrophils, and C-reactive protein, where a two-sample t-test was undertaken.
†Mean and standard error.
‡No statistical significance after adjustment for comorbidities and other variables.
§Yes is positive (≥80 pg/mL).
BAL: broncho-alveolar lavage; DMARD: disease-modifying anti-rheumatic drug; WBC: white blood cells.
The majority of the patients diagnosed with PCP did not have any malignancy (55%; Table 1). Among patients with a prior diagnosis of neoplasia, 71% and 29% had solid organ tumour and haematological malignancy, respectively. Patients with solid organ tumours made up 33% of all patients in this study, compared to patients with haematological malignancies, who made up 15% of the study group.
Autoimmune/rheumatological conditions were the most common diseases among patients with PCP without known cancer diagnosis (83%), followed by patients with COPD (61%), diabetes (33%), solid organ transplant (11%), and hepatitis C (6%; Figure 1). The majority of patients who had no prior diagnosis of malignancy were on disease-modifying anti-rheumatic drugs and steroids.
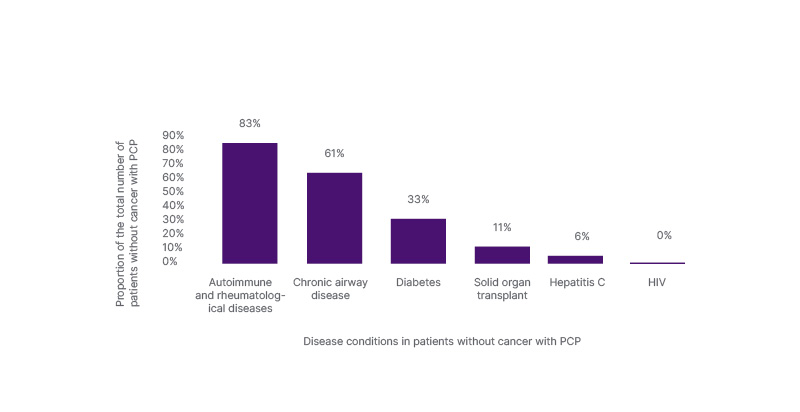
Figure 1: Prevalent conditions in patients with Pneumocystis jirovecii pneumonia without a cancer diagnosis.
PCP: Pneumocystis jirovecii pneumonia.
Chest X-ray was the most performed radiology procedure (91%), with 70% of the chest X-rays showing new abnormalities. Only 48% of the patients had a non-contrast chest CT scan or high-resolution chest CT, and all were reported as abnormal. Findings on the CT scans were heterogeneous, ranging from bilateral reticular interstitial opacification, to extensive diffuse patchy ground glass opacities, bilateral pleural effusions, mosaic interstitial changes, bilateral upper lobe interstitial thickening, atelectasis, ground-glass nodularity, etc.
Seventy-six percent (19/25) of the patients had an initial procalcitonin (PCT) level <0.5 ng/mL (Table 1). β-D-glucan (BDG) was positive in 45% (9/20) of the patients (assay cut-off for positive report was ≥80 pg/mL). There was no difference in in-hospital fatality between patients with positive BDG and those with negative results. Similarly, there was no difference in fatality rates between patients with PCT <0.5 ng/mL compared to those with ≥0.5 ng/mL. Conversely, patients who died in hospital had a statistically significant higher total white blood cell and neutrophil count. However, statistical difference was not observed following a multivariate logistic regression that adjusted for age and underlying medical conditions.
Sputum induction was performed in 18% of the cohort and a positive identification of the P. jirovecii DNA was noted in 50% of the patients. Conversely, bronchoscopy with bronchoalveolar lavage (BAL) was only undertaken in 39% of the patients. Detection of pathogen occurred in 85% of those patients. Forty-two percent of the patients had neither bronchoscopy nor sputum induction.
Microbiological diagnosis of PCP made based on positive identification of the causative organism for PCP, P. jirovecii, in sputum or BAL sample was made in 42% of the patients. Therefore, most patients (58% of the study population) were diagnosed with PCP based on either clinical and/or radiological grounds. All patients were treated with high dose cotrimoxazole for 21 days, except for the patients who died prior to completing the antibiotics. All the patients were additionally treated adjunctively with prednisolone.
In-hospital fatality rate was 27% among all patients with PCP. Death was higher in patients without a cancer diagnosis (39%) compared to patients with known cancer diagnoses (13%). This difference in death rate was not statistically significant (Fisher’s exact test, one-tailed p=0.105; Table 1).
DISCUSSION
PCP, a disease known historically to affect patients with AIDS,10 now has an increasing prevalence among people with diagnoses of malignancy, autoimmune diseases, and other conditions requiring long-term use of immunosuppressive medications, such as in COPD.11
In the authors’ study, the diagnosis of PCP was more common among patients without cancer (55%), with the majority of them having autoimmune and rheumatological conditions, followed by COPD and diabetes (Figure 1). Their findings support the rising incidence of PCP among patients without HIV. In a study by Matsumura et al.,12 the team retrospectively analysed the clinical phenotypes of patients without HIV who had a PCP diagnosis. Of 82 patients, 61% were reported to have an underlying inflammatory disease such as rheumatoid arthritis, systemic lupus erythematosus, vasculitis, inflammatory myopathy, etc. In the same study, 21%, 15%, and 22% had solid organ malignancy, haematological malignancy, and pulmonary disease (other than lung cancer), respectively.12 Conversely, in a retrospective multicentre study by Lécuyer et al.,13 18.1% and 21% of the patients were recorded to have immune-mediated inflammatory disease and COPD, respectively.13 Furthermore, a 2-year prospective cohort study looking at PCP in an ICU reported that the majority of patients (66/158; 42%) had a diagnosis of solid tumour or haematological malignancy.14 This result is similar to the authors’ finding that 45% of their study population had a diagnosis of a malignancy; however, PCP was more prevalent in patients without malignancy in the authors’ study.
The diagnostic gold standard for PCP is the demonstration of the organism in a deep respiratory sample from either induced sputum or bronchoscopy with BAL.15 The authors’ results showed that this was performed infrequently. Although the reason for this was not explored in their cohort, it has been reported that it is often difficult to obtain BAL samples in patients without HIV who have severe and rapidly progressive disease.16,17 In the authors’ study, among the patients who underwent a sputum induction or bronchoscopy with BAL, the majority had positive tests for PCP.
Given the challenges and potential delays in obtaining a bronchoscopy, the use of adjunctive biochemical markers for the early identification of patients with PCP is warranted. BDG is a frequently used test to predict the likelihood of PCP in a patient.15 In the authors’ cohort, BDG was positive (≥80 pg/mL) in 45% of the patients with a test result. Conversely, in a study by Iikuni et al.,18 BDG was reported to be elevated in 78% of 18 patients without HIV with a PCR-based diagnosis. The role of BDG in predicting mortality in patients with PCP has been explored in a few studies with mixed results.12,18-20 In the authors’ study, there was no difference in in-hospital mortality between patients with raised BDG compared to those without. BDG is a cell wall component of P. jirovecii; therefore, the level of BDG may reflect the organism burden directly, not the severity of PCP.21 More studies are required to further clarify the role of BDG in the diagnosis and prognosis of PCP in patients without HIV.
Similarly, serum PCT could represent a useful adjunctive test in the diagnosis of PCP. In the authors’ study, the majority of patients with a diagnosis of PCP had a low PCT level (<0.5 ng/mL; 19/25 patients). There was no statistically significant difference in inpatient mortality between patients whose PCT level was below or above the threshold. The role of PCT in supporting the diagnosis of PCP in patients without HIV is not well reported.
In contrast, several studies have explored the diagnostic utility of PCT in patients with HIV and PCP. For example, Nyamande et al.22 reported significantly lower PCT levels in patients with HIV and PCP compared to those with bacterial pneumonia. In another study, involving lung transplant patients, Zeglen et al.23 demonstrated a correlation between bronchial tree colonisation by PCP and increased serum PCT. Conversely, in a study by Salerno et al.,24 there was no difference in PCT levels between patients who were PCP positive and those who were PCP negative in a HIV population. The group reported median serum PCT levels of 0.16 ng/mL (interquartile range: 0.06–0.44) and 0.13 ng/mL (interquartile range: 0.05–0.52) for patients who were PCP positive and negative, respectively, which did not reach a statistically significant difference. The group concluded that the plausible cause of lack of statistical significance might be due to unmeasured bacteria presence in the alveolar space. Studies exploring the diagnostic role of serum PCT in patients who are HIV negative are needed.
All-cause in-hospital fatality of patients with PCP remains high. In patients who are HIV positive, highly active antiretroviral therapy may contribute to more rapid control of the infectious process through faster recovery of the immune system. In contrast, HIV-negative patterns of immunosuppression and long-term steroid therapy could be associated with an inappropriate inflammatory response in cases of high fungal load.25 The authors’ study reported an in-hospital fatality rate of 27%, which is slightly lower compared to other studies reporting a fatality rate of 30.6–84.2%.6-8 Furthermore, they observed that patients without cancer had a higher in-hospital mortality than patients with known cancer, but this difference in fatality did not reach a statistical significance (Fisher’s exact test, one-tailed p=0.105; Table 1). The lack of statistically significant difference in mortality in the authors’ study could be due to a lack of adequate statistical power.
A higher hospital death rate was also reported in patients without cancer in a French multicentre prospective cohort study. In this study, Kamel et al.14 reported an in-hospital fatality rate of 37.9% for patients with haematological or solid organ malignancy, 10.3% in patients with HIV infection, 57.1% in solid organ transplant patients, and 40.8% in ‘others’, with the between group rate differences reaching statistical significance (p=0.005). The ‘others’ were made up of patients with diverse inflammatory/autoimmune diseases, including patients on corticosteroid therapy. This study therefore demonstrates higher mortality risk among patients with PCP and no prior malignancy, especially those with rheumatological disease and organ transplant, compared to patients with a cancer diagnosis.
A univariate analysis in the authors’ study revealed that the risk factors for inpatient death in patients with PCP without HIV were advancing age, raised total white blood cell count, and neutrophils (Table 1). However, following a multivariate logistic regression (adjusting for underlying diseases and other confounding variables), the authors’ study did not identify factors that predict in-hospital fatality. In published systematic reviews and meta-analyses of risk factors for mortality in patients with PCP without HIV, age, underlying pulmonary diseases at diagnosis of PCP, solid tumours, cytomegalovirus co-infection, lactate dehydrogenase, lymphocyte count, invasive ventilation during hospitalisation, and pneumothorax were reported to predict mortality.26
The main risk factors for developing PCP are deficiencies in cellular immunity and the use of immunosuppressive agents, especially corticosteroids.27-29 The evolving epidemiology of non-HIV PCP revealed in the authors’ study has important implications for prophylaxis strategies. Among patients without HIV, the routine use of PCP prophylaxis in haematological malignancy and transplant patients explains the higher prevalence of rheumatological conditions, as noted in the authors’ study. Similarly, Matsumura Y et al.12 noted that nearly two-thirds of patients with PCP had inflammatory diseases and fewer patients had haematological malignancies or transplantation.
The EULAR currently recommends prophylaxis against PCP in patients with autoimmune inflammatory rheumatic diseases in whom high doses of glucocorticoids are used, especially in combination with immunosuppressants.9 The EULAR cautioned that there was currently a lack of robust evidence of the benefits of prophylaxis, as most studies do not focus on a specific autoimmune inflammatory rheumatic disease. Therefore, it was not possible to make recommendations for PCP prophylaxis in individual diseases. Thus, there is a strong clinical need for practical and nuanced risk assessment tools that incorporate age, comorbidity burden, and disease-specific factors to support robust and timely identification of high-risk patients who fall outside traditional prophylaxis criteria. This approach would enable more targeted prophylaxis strategies that optimise the balance between preventing PCP and mitigating unnecessary antimicrobial exposure. The most commonly used prophylaxis for PCP is a combination of trimethoprim and sulfamethoxazole (480 mg/day or 960 mg three times a week).
LIMITATIONS
This study presents several limitations due to its retrospective nature. The first limitation is the small sample size, which potentially affected the strength of the conclusions that could be drawn from the study and reduced the statistical power to determine a clinical relationship between underlying diseases and in-hospital mortality. However, the descriptive data remain of interest.
Furthermore, due to reliance on ICD coding, there could be a potential underreporting of PCP cases, especially if appropriate diagnostic codes were not assigned.
The absence of detailed information on immunosuppressive regimens and duration of therapies may have influenced derived outcomes. Unfortunately, the collection of extensive data on immunosuppressants is limited by the accessible information on the clinical care system. Similarly, potential confounding factors that influence susceptibility to PCP were not fully accounted for, such as nutritional state, smoking history, and severity of underlying diseases.
The disparity in the diagnostic criteria applied to the cases is an important limitation. More than half of the patients in the authors’ study did not have a positive identification of the causative organism in a respiratory sample. Unfortunately, this reflects current challenges faced in the diagnostic work up of patients suspected of having PCP.
CONCLUSION
The authors’ study highlights a rising incidence of PCP in patients without HIV. This trend is linked to the increased use of immunosuppressive therapies to treat autoimmune and rheumatological conditions. Furthermore, the increase in cancer diagnosis and treatment also contributes to the growing cases of PCP. In patients with PCP, in-hospital fatality remains high. Therefore, it is important that clinicians have a low threshold of clinical suspicion of PCP in at-risk clinical groups, such as individuals with known rheumatological disease, cancer, or those who are on long-term immunosuppressive therapies. Future studies are warranted to develop more comprehensive risk assessment tools that reflect current changes in the population at risk of PCP.







