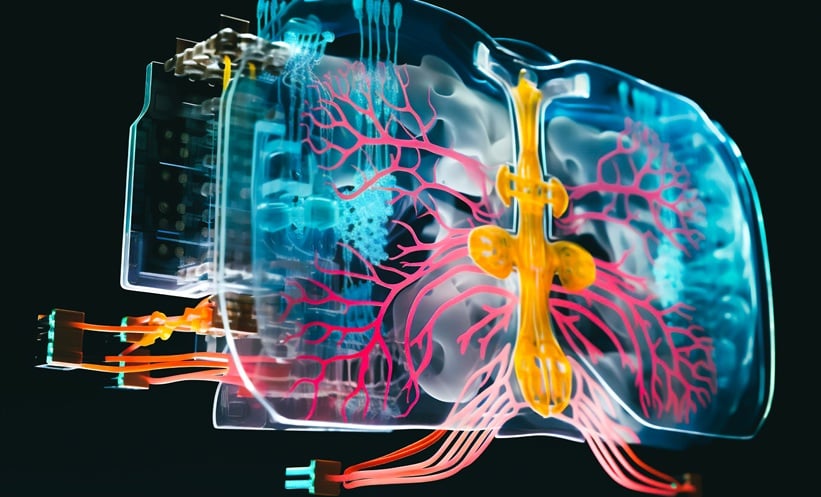
RESEARCHERS have developed a cutting-edge human lung-on-chip system, called iLoC, that could transform how clinicians and scientists study respiratory infections, including tuberculosis (TB). Unlike traditional 2D cultures or animal models, this platform recreates a 3D alveolar environment using human induced pluripotent stem cells (iPSCs) from a single donor, providing an immunocompetent and experimentally accessible model. The device integrates type I and II alveolar epithelial cells, vascular endothelial cells, and macrophages under air-liquid interface and mechanical stretching conditions that closely mimic human lung physiology.
Tuberculosis Infection Recapitulated in Human Cells
Using the iLoC, the team explored early-stage Mycobacterium tuberculosis (Mtb) infection. They found that both alveolar macrophages and epithelial cells became infected, yet these cells were largely non-permissive to bacterial replication, unlike findings in standard 2D cell cultures. Notably, stochastic clusters of macrophages underwent necrosis, supporting limited Mtb replication. These results provide novel insight into the heterogeneous nature of alveolar infection and the role of macrophages in TB pathogenesis.
Autophagy Gene ATG14 Shapes Immune Response
By introducing genetically engineered ATG14-deficient macrophages into the iLoC, researchers observed increased macrophage cell death both at baseline and after Mtb infection. This indicates that autophagy, particularly through ATG14, is a critical regulator of alveolar macrophage survival and may influence tissue inflammation and damage during infection. Cytokine analysis showed elevated IL-8 and IP-10 in standard iLoC but not in the autophagy-deficient model, highlighting the role of autophagy in modulating immune signalling and immune cell recruitment.
Implications for TB Research and Therapeutics
The iLoC represents a physiologically relevant, genetically tractable platform for studying human alveolar biology, early TB infection, and host-pathogen interactions. Unlike animal models, it allows for human-specific observations and controlled genetic manipulation, offering a new avenue for investigating TB pathogenesis, inflammatory responses, and testing potential therapeutics. The study also highlights differences in alveolar versus blood-derived macrophage responses, emphasising the importance of context-specific models in respiratory research.
This pioneering work provides clinicians and researchers with a powerful tool to bridge the gap between molecular mechanisms of infection and patient-relevant lung pathology, advancing precision medicine for TB and other respiratory diseases.
Reference
Luk CH et al. Autologous human iPSC-derived alveolus-on-chip reveals early pathological events of Mycobacterium tuberculosis infection. Sci Adv. 2026;12(1):eaea9874.