Meeting Summary
Non-cystic fibrosis bronchiectasis (NCFBE) is often under-recognized and misdiagnosed, and is associated with significant clinical and economic burden. This article highlights two presentations from the American Thoracic Society (ATS) 2025 International Conference, which took place in San Francisco, California, USA, between 17th–21st May 2025, highlighting real-world evidence (RWE) data on treatment patterns, complications and healthcare resource utilization (HCRU) associated with NCFBE.
Ruxana T. Sadikot, Professor and Chief of the Pulmonary, Critical Care and Sleep Medicine Division at the University of Nebraska Medical Center (UNMC), Omaha, USA, and Sunjay R. Devarajan, Assistant Professor of Internal Medicine, Pulmonary and Critical Care at Baylor College of Medicine, Houston, Texas, USA, presented real-world evidence of treatment patterns and costs associated with HCRU in NCFBE.
Non-Cystic Fibrosis Bronchiectasis
Bronchiectasis is a chronic and progressive inflammatory lung disease, characterized by permanent and abnormal dilatation of the bronchi and accompanied by cough, sputum production, and recurrent bronchial infection and exacerbations.1,2 Bronchiectasis that is not associated with cystic fibrosis is known as NCFBE.3
The pathophysiology of bronchiectasis has been described as a vicious vortex, driven by inflammation, chronic airway infection, impaired mucociliary clearance, and progressive lung damage.3,4 Neutrophils are the primary inflammatory cells in patients with bronchiectasis and are believed to be key drivers in disease severity and progression.3-8 Bronchiectasis is associated with high disease burden on patients and healthcare systems, presenting with recurrent exacerbations and progressive lung function decline, increased hospitalizations, and worsening health-related quality of life.9-13 International guidelines for bronchiectasis recommend a multi-modal approach focusing on controlling symptoms and infection, reducing exacerbations, enhancing mucociliary clearance, and treatment of etiologies and comorbidities.9,14,15 There are no US guidelines.The primary management goals of bronchiectasis focus on symptom control and reducing exacerbation frequency to improve health-related quality of life.14,15 However, challenges arise due to the clinical heterogeneity and limited treatment options.16 This highlights the need for data on treatment patterns and clinical outcomes to enhance understanding of bronchiectasis management practices. Here, this article focuses on new data presented at the ATS that highlights insights on treatment patterns, complications, and hospitalizations in NCFBE.
Treatment Patterns and Outcomes Among Patients with Non-Cystic Fibrosis Bronchiectasis
Lead Author/Presenter: Ruxana T. Sadikot
The clinical and economic burden of NCFBE on patients and healthcare systems is significant,16 but not thoroughly understood, especially regarding HCRU.
Findings were presented from a retrospective cohort study using de-identified claims from Optum’s Market Clarity database.16 The study included patients diagnosed with NCFBE in the USA between January 1st, 2017–March 31st, 2020.16 Eligible patients were those ≥12 years old with either ≥2 outpatient claims for bronchiectasis ≥30 days apart or ≥1 inpatient claim, excluding those with cystic fibrosis. Continuous enrollment was required for ≥12 months before (baseline) and ≥24 months after (follow-up) the first bronchiectasis claim.16 The analysis identified 12,018 people with NCFBE (mean age 68.5±13.2 years; 67% female).16
Commonly Prescribed Medications for Patients with Bronchiectasis
During the 2-year follow-up period, the commonly prescribed medications included corticosteroids, short-acting β-agonists antibiotics, and long-acting β-agonists. During the follow-up period, treatment patterns (for any duration), identified that around half of patients received one or more medications (Figure 1).16
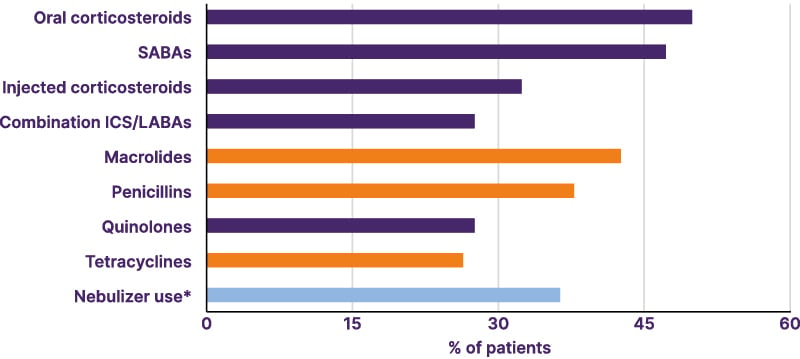
Figure 1: Most common treatments of any duration for bronchiectasis (N=12,018; any time during follow-up using medical and pharmacy claims).16
*General nebulizer-device use, excluding inhalers and distinct from antibiotic or bronchodilator use.
ICS: inhaled corticosteroids; LABA: long-acting β-agonist; SABA: short-acting β-agonist.
Notably, 37.5% were prescribed long-acting bronchodilators for ≥6 months. Injectable corticosteroids were used by 32.6% of the patients, with 28.4% prescribed inhaled corticosteroids/long-acting β-agonist combination inhalers. Bronchodilators were used for a mean duration of 4.5 months.16 Macrolides were the most common first-line antibiotics prescribed (25.7%), with a mean of 7 months.16 Of these patients, 94% discontinued their treatment.17 During follow-up, nearly half of the patients switched back to a macrolide (49.7%); the remaining were prescribed alternative antibiotics such as penicillin (49.5%), quinolones (62.1%), and cephalosporins (63.0%).16 Long-term use of macrolides (≥6 months) was used in 9.4% of patients, with a mean duration of treatment of 1.2 months, compared to approximately 12 days for other antibiotics.16
Complications and Mortality Associated with Bronchiectasis
Sadikot reported that complications associated with NCFBE included respiratory failure, heart failure, having a lung transplant, and experiencing ≥1 bronchiectasis exacerbation. Approximately one-fifth of patients (22%) experienced respiratory failure over the 2-year follow-up period (Table 1).16
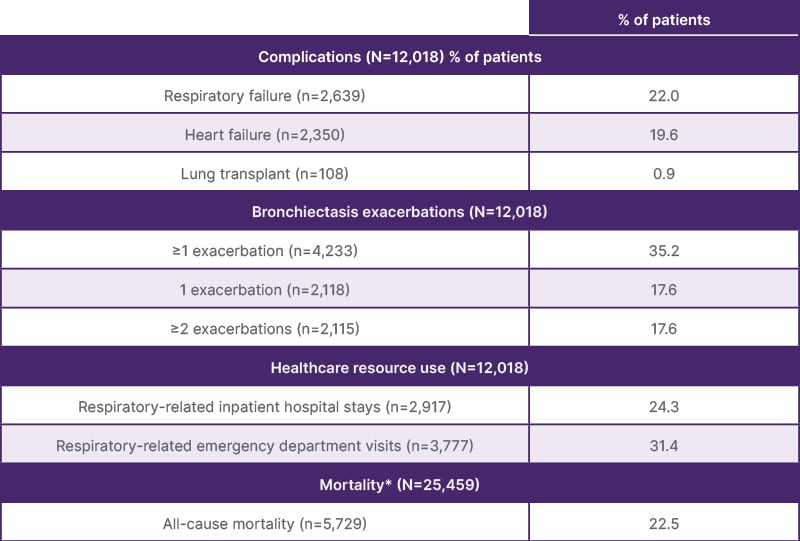
Table 1: Complications and all-cause mortality during follow-up.17
*Continuous enrollment or clinical activity were not required during follow-up.
Regarding HRCU, data indicated that 24.3% of patients (n=2,917) experienced respiratory-related inpatient hospital stays, and 31.4% (n=3,777) had emergency department visits (Table 1).16 The all-cause mortality rate during follow-up was 22.5% (n=5,729/25,459).16
Sadikot stated the complexity of treating NCFBE due to its heterogeneity. Frequent switching of antibiotics, reliance on multiple symptom-based therapies and steroids, along with ongoing exacerbations, suggest current approaches often fall short of effectively managing bronchiectasis.16
Hospitalizations Burden and Risk of Readmissions in Non-Cystic Fibrosis Bronchiectasis
Lead Author: Sunjay R. Devarajan
Presenter: John Fastenau
Another RWE study described a retrospective analysis to understand the inpatient journey and readmission risk in patients hospitalized with NCFBE.18
Using the USA PINC AI™ Healthcare database, 73,656 patients aged ≥12 years (mean [SD] age 71.8±14.7 years; 75.3% White; 58.3% female), hospitalized between January 1st, 2018–June 30th, 2022, with a primary or secondary diagnosis of bronchiectasis were identified (comorbid COPD and asthma were permitted).18 Notably, 92.3% were admitted on an urgent or emergency basis.17 Patients with a primary diagnosis of COPD or asthma, and all those with cystic fibrosis, were excluded from the analysis.18
At index hospitalization, 39.8% of patients had an existing respiratory infection, 29.5% had respiratory failure, and 85.6% had a pulmonary exacerbation.19 The most common treatments during hospitalizations were antibiotics (83.9%), bronchodilators (76.8%), and corticosteroids (68.6%).18 Additionally, 34.6% of patients required mechanical ventilation.19
The average (SD) length of index hospital stay was 7.1±9.4 days, costing on average 22,355±45,160 USD. Approximately 17% required intensive care, averaging 16,375±35,847 USD in costs. Following the index hospitalization, 5.9% of patients died.18
Also, Fastenau reported that post-discharge, most patients had significant post-acute care needs, with over half (53.8%) requiring special discharge plans; 23.3% were discharged to specialized healthcare facilities for long-term, around-the-clock care, and 21.9% received home healthcare.18 Patients were also likely to be readmitted to hospital, resulting in higher healthcare costs; 22.6% were readmitted within 90 days of discharge, for on average 7.9±9.6 days, and costing 24,576±41,189 USD.18
Multivariable logistic regression (Figure 2) indicated that all-cause readmission risk was associated with prior hospitalizations, a history of COPD or asthma, and longer hospital stays (all p<0.001).18
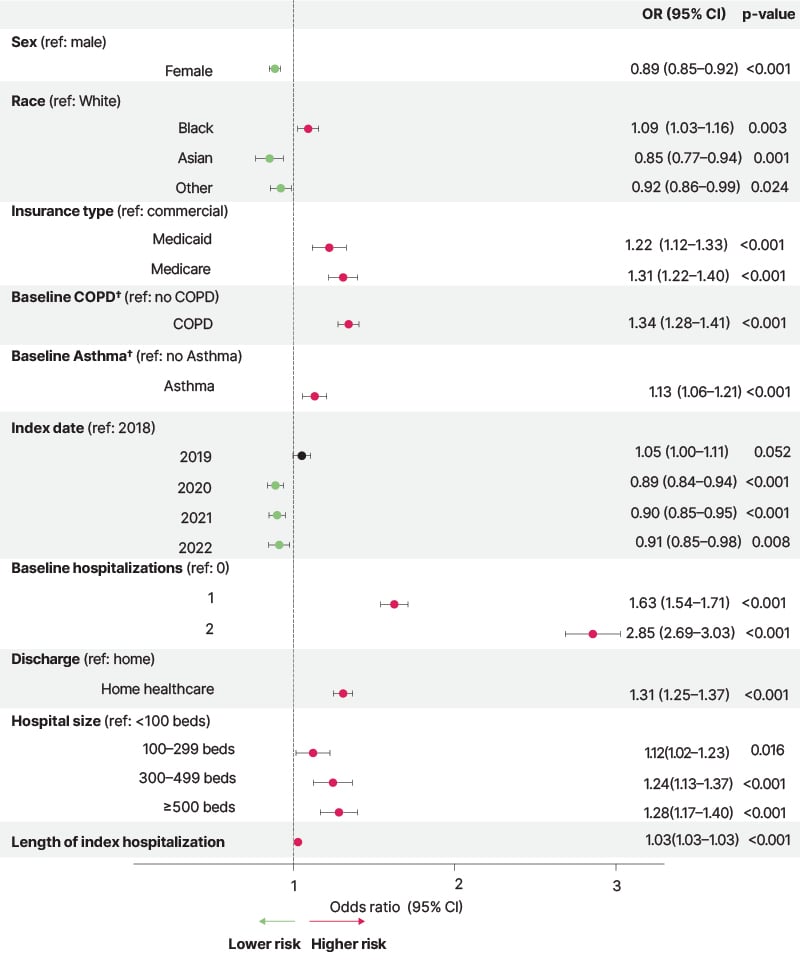
Figure 2: Risk factors for 90-day hospital readmission in patients hospitalized with non-cystic fibrosis bronchiectasis*.18
*Multivariate logistic regression modeling was performed. From the total population (N=73,656), 4,380 patients were excluded from modeling because they did not have data available in one or more predictors or died at index hospitalization. Additional variables included in the model that were nonsignificant (CI did not cross 1) were the
number of pulmonary exacerbations at baseline, ethnicity, and age.
†Patients with a diagnosis code for COPD or asthma in the secondary position in a claim.
Fastenau stated that the high healthcare costs associated with NCFBE (hospitalization, readmission, and need for continuous medical care) highlight the need for improved continuity of care post-discharge to reduce readmission rates.18
Conclusion
These presentations highlight considerable variation and suboptimal management strategies for NCFBE. Frequent switching of antibiotics, reliance on multiple symptom-based therapies and steroids, along with ongoing exacerbations, suggest current approaches often fall short of effectively managing bronchiectasis. Exacerbations and complications are contributing to high hospital readmission rates and direct medical costs. This review highlights gaps in the current management of NCFBE and underscores the need for more effective approaches to reduce clinical complications and healthcare burden.
MED-ALL-BE-00039







