Meeting Summary
Dupilumab is a fully human monoclonal antibody that blocks the shared receptor component for IL-4 and IL-13, key and central drivers of Type 2 inflammation in multiple diseases. Its efficacy in diseases involving Type 2 inflammation has been demonstrated across multiple Phase III clinical trials, including severe asthma, uncontrolled COPD, and chronic rhinosinusitis with nasal polyps (CRSwNP). This article summarises data from a selection of posters on dupilumab in asthma and COPD, which were presented at the 2025 European Respiratory Society (ERS) Congress in Amsterdam, the Netherlands, and CHEST Annual Meeting in Chicago, Illinois, USA.
Arnaud Bourdin from the University of Montpellier, France, presented data from the Phase II LIBERTY ABPA AIRED study, the first randomised, placebo-controlled trial to demonstrate the benefit of dupilumab in patients with asthma and allergic bronchopulmonary aspergillosis (ABPA). In this patient population with substantial unmet need, dupilumab significantly improved lung function and quality of life (QoL), while reducing systemic corticosteroid (SCS) use. In interim results from the ProVENT study, disclosed by Henrik Watz from Velocity Clinical Research, Ahrensburg, Germany, real-world treatment with dupilumab sustainably improved lung function, asthma control, and QoL, with 58% of patients achieving clinical remission after 2 years. Brian Lipworth from the University of Dundee, UK, showcased results from the head-to-head EVEREST study, in which dupilumab delivered improvements in asthma control and lung function, including measures of small and large airway function and air trapping, compared to omalizumab in patients with CRSwNP and uncontrolled asthma.
In the COPD space, Nicola Hanania from Baylor College of Medicine, Houston, Texas, USA, presented results from post-hoc analysis of the pivotal BOREAS and NOTUS trials, showing that COPD exacerbations had less impact on lung function in patients receiving dupilumab treatment. A further post-hoc analysis of the BOREAS and NOTUS studies, presented by Mona Bafadhel from King’s College London, UK, demonstrated that dupilumab significantly reduced the risk of severe exacerbations requiring hospitalisation and/or emergency department (ED) visits by 38% versus placebo.
Dupilumab Efficacy in Patients with Asthma and Allergic Bronchopulmonary aspergillosis
ABPA is driven by Type 2 inflammation and characterised by hypersensitivity to the fungus Aspergillus fumigatus following colonisation of the airways in patients with asthma.1-3 This rare and progressive lung disease affects 2.5% of patients with asthma globally, and is characterised by more severe clinical outcomes than asthma without ABPA.2,4 The mainstay of current treatment for ABPA is SCS, plus adjunctive antifungals, which are associated with adverse effects and also fail to address the underlying immunological basis of the condition.5-7
Dupilumab is already approved as add-on therapy for severe asthma with Type 2 inflammation.8 The objective of the Phase II LIBERTY ABPA AIRED study (NCT04442269), presented by Bourdin at ERS 2025, was to evaluate the efficacy and safety of dupilumab in patients with asthma and ABPA.7 In total, 62 patients aged ≥12 years with uncontrolled asthma and meeting clinical criteria for ABPA were randomised to receive dupilumab 300 mg (n=35) or placebo (n=27) every 2 weeks for 24–52 weeks. The criteria used to define ABPA was elevated serum total IgE >1,000 IU/mL; IgE levels ≤1,000 IU/mL were acceptable if all three supportive criteria were met:7
- For patients on oral corticosteroids, blood eosinophil count >500 cells/μL; for patients not on oral corticosteroids, ≥300–≤500 cells/μL was acceptable with a historical result of >500 cells/μL within 12 months of screening.
- Serum precipitating or IgG antibodies to A. fumigatus.
- Documented radiological findings consistent with ABPA by historical chest X-ray, chest CT, or MRI within the previous 18 months or at screening.
Uncontrolled asthma was defined as ≥1 severe respiratory exacerbation requiring treatment with SCS, or hospitalisation, or treatment in ED/urgent care within the past 12 months; or receiving long-term, low-dose SCS. Stable background therapy for APBA and asthma was permitted during the course of the study.7
The primary endpoint of the LIBERTY ABPA AIRED study was change from baseline in pre-bronchodilator forced expiratory volume in 1 second (FEV1) at Week 24. Key secondary and exploratory endpoints included: QoL, as evaluated by change from baseline in St. George’s Respiratory Questionnaire (SGRQ) total score; percentage reduction from baseline and discontinuation of SCS use; annualised rate of severe respiratory exacerbations; and percentage change from baseline in total and A. fumigatus-specific IgE. As this study was conducted during the COVID-19 pandemic, the number of patients enrolled was smaller than originally planned, with the resulting statistical analysis therefore yielding nominal p values, with the exception of the primary endpoint.7
Baseline characteristics were reflective of the high disease severity of enrolled patients. The mean pre-bronchodilator FEV1 in all study participants was 1.90 L, and the mean percentage predicted FEV1 (ppFEV1) was low at 68.3%. Over 30% of patients (n=19) had experienced two or more asthma exacerbations in the 12 months prior to screening. Mean patient age was 59.4 years, and the majority (62.9%) were female.7
In this Phase II study, dupilumab improved pre-bronchodilator FEV1 over the 24- to 52-week treatment period compared to placebo, with benefits seen from as early as Week 2 of treatment (Figure 1).7
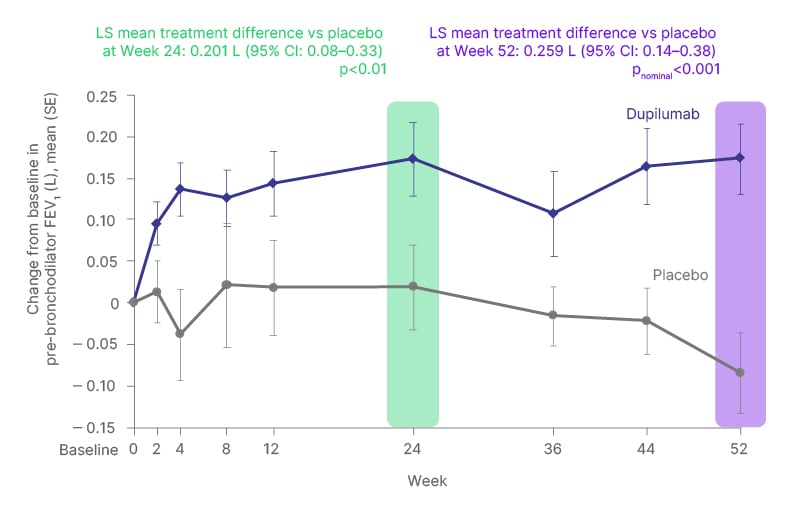
Figure 1: Dupilumab improved pre-bronchodilator forced expiratory volume in 1 second over the 24- to 52-week treatment period versus placebo in patients with asthma and allergic bronchopulmonary aspergillosis.7
FEV1: forced expiratory volume in 1 second; LS: least squares; SE: standard error; vs: versus.
These improvements in lung function with dupilumab were associated with improvements in patient QoL over the treatment period. From baseline to Week 52, SGRQ total score decreased by a total of 16.0 points in dupilumab-treated patients versus placebo (pnominal<0.01). Lower scores indicate better QoL, with a change of 4 points considered to be the minimal clinically important difference.7
Dupilumab was also able to reduce patients’ reliance on SCS treatment during the course of the study. A higher proportion of patients on dupilumab versus placebo were able to reduce their SCS dose by 50% or more and/or completely stop SCS treatment. By Week 24, 85.7% of patients on dupilumab had SCS dose reductions ≥50% compared to 28.6% on placebo (pnominal<0.01). Similarly, 71.4% of dupilumab-treated patients had complete cessation of SCS by Week 24, compared to 14.3% on placebo (pnominal<0.05).7
In terms of other efficacy endpoints, treatment with dupilumab reduced the annualised rate of severe respiratory exacerbations by 55.2% versus placebo (pnominal=0.0627). Adjusted annualised rates (95% CI) were 0.695 (0.35–1.36) versus 1.551 (0.75–3.22) events per person-year for the two arms, respectively. Treatment with dupilumab also led to a decrease in total and A. fumigatus-specific IgE levels compared to placebo. At Week 52, patients receiving dupilumab had a 49.1% mean reduction from baseline in A. fumigatus-specific IgE compared to a 12.6% mean increase from baseline in the placebo group.7
The safety profile of dupilumab in this study in patients with asthma and ABPA was consistent with the known safety profile. Drug-related treatment-emergent adverse events (TEAE) occurred in 37.1% and 18.5% of patients in the dupilumab versus placebo arms, respectively. TEAEs leading to study drug discontinuation occurred in 2.9% of dupilumab-treated patients compared to 11.1% on placebo. Most TEAEs associated with dupilumab were mild or moderate in severity. There were no drug-related serious TEAEs or drug-related deaths in either study arm.7
Overall, these findings from the Phase II LIBERTY ABPA AIRED study demonstrated that treatment with dupilumab significantly improves lung function, while additionally improving QoL and reducing SCS use in patients with asthma and ABPA. Dupilumab also reduced severe respiratory exacerbations and total A. fumigatus-specific IgE levels in these patients.7
This study was the only industry-sponsored submission to win a best abstract award at ERS 2025, reflecting the importance of the findings in addressing unmet needs in the asthma plus ABPA setting.
Real-World Outcomes After 2 Years of Dupilumab Therapy for Severe Asthma: The ProVENT Study
Evidence from RCTs has amply demonstrated dupilumab’s efficacy in improving clinical outcomes in patients with asthma.9-13 However, real-world data remain important to supplement evidence from RCTs and confirm that the effectiveness and safety of dupilumab is reflected in everyday clinical practice use.
ProVENT (NIS-Nr: 514; study code: OBS16379) is an ongoing, prospective, non-interventional, single-arm, 3-year study of real-world dupilumab therapy for severe asthma being conducted in Germany, Austria, and Switzerland.14 Its aim is to describe the real-world effectiveness of dupilumab treatment and clinical remission rates up to 2 years. Since February 2020, ProVENT has included 399 patients aged 12 years or over with severe uncontrolled asthma initiating dupilumab treatment in a routine clinical setting.14,15
Results of this interim analysis of the ProVENT study, presented by Watz at ERS 2025, considered 259 patients in the full analysis set, including 185 patients who had reached 1 year and 100 patients who had completed 2 years of therapy.15 The mean age of these patients was 56 years and 53% were female. Study assessments included biomarker levels, severe exacerbation events, asthma control, and QoL, which were measured at baseline and every 3 months until Month 24. The specific biomarkers evaluated in the study were blood eosinophil counts, total IgE, and fractional exhaled nitric oxide. Lung function was assessed by pre-bronchodilator FEV1 and ppFEV+1, and QoL by 5-item Asthma Control Questionnaire (ACQ-5), Asthma Control Test (ACT), and standardised Asthma Quality of Life Questionnaire (AQLQ[S]) scores. The proportion of patients achieving clinical remission with real-world dupilumab treatment was also calculated at Years 1 and 2. Clinical remission criteria were defined as no oral corticosteroid use; no exacerbations; ACT ≥20 or ACQ-5 ≤1.5; and pre-bronchodilator FEV1 ≥80% or reduced by ≤5% versus baseline.15
Patients treated with real-world dupilumab in the ProVENT study showed decreases in levels of disease biomarkers. Both baseline fractional exhaled nitric oxide (42 ppb) and total IgE (160 IU/mL) normalised rapidly at Month 3 after dupilumab initiation (18 ppb and 76 IU/mL, respectively), and remained low until Year 2 (22 ppb and 28 IU/mL, respectively). Dupilumab treatment also decreased blood eosinophil levels from 320 cells/μL at baseline to 184 cells/μL at Year 2.15
These decreases in key biomarker levels were accompanied by improvements in lung function, asthma control, and QoL with dupilumab treatment over the first 2 years of the ProVENT study. Patients’ asthma control and QoL steadily improved from baseline to Year 2, as evidenced by a drop in ACQ-5 score from 2.6 to 0.9 points, and an increase in ACT score from 14 to 22 points. Lung function quickly improved with real-world dupilumab treatment and then stabilised. Pre-bronchodilator FEV1 increased from a baseline level of 2.08 L to 2.39 L by Month 3, and remained at 2.36 L at Year 2.15
Real-world dupilumab treatment in the ProVENT study also had a notable impact on asthma exacerbations in patients with available data. At Year 2 of the study, 89% of patients were free of asthma exacerbations, despite having experienced an average of two severe exacerbations in the 2 years prior to starting dupilumab treatment.15
Importantly, over half of patients treated with dupilumab in the real-world setting were also able to achieve clinical remission from asthma, an evolving target in disease management. Overall, 56% of patients at Year 1 and 58% at Year 2 met all four criteria for clinical remission in the ProVENT trial. These rates of clinical remission are higher than those seen in clinical trials of dupilumab, potentially reflecting the less severe patient population receiving treatment in the real world.9-13,15
Collectively, these interim results from the ProVENT study confirm the effectiveness of dupilumab when used in the real-world clinical practice setting and support the efficacy seen in clinical trials in asthma.9-13,15 Real-world dupilumab treatment improved biomarker levels, lung function, asthma control, and QoL over 2 years in patients with severe uncontrolled asthma. Importantly, 89% of patients were exacerbation-free after 2 years, and 58% achieved the key target of clinical remission.15
Dupilumab Versus Omalizumab: Greater Lung Function Improvements in Patients with Chronic Rhinosinusitis with Nasal Polyps and Uncontrolled Asthma with Dupilumab – The EVEREST Study
Type 2 inflammation is involved in the underlying pathophysiology of both CRSwNP and asthma, and patients in whom these two conditions coexist typically have higher disease severity and an increased overall disease burden.16 Dupilumab (a fully human monoclonal antibody that blocks the shared receptor component for IL-4 and IL-13) and omalizumab (a humanised monoclonal antibody targeting IgE), are two biologic agents approved for the treatment of inadequately controlled CRSwNP and uncontrolled moderate-to-severe asthma.8,17,18
EVEREST (NCT04998604) was a multicentre, randomised, double-blind, Phase IV clinical trial that evaluated the efficacy and safety of dupilumab versus omalizumab in patients with severe CRSwNP and coexisting asthma. Initial findings from the EVEREST trial have already been reported, and showed that dupilumab significantly improved CRSwNP-related outcomes over omalizumab.19 This analysis, presented by Lipworth at CHEST 2025, focused specifically on changes in lung function parameters and asthma control in patients treated with dupilumab versus omalizumab.20
The EVEREST study included patients aged 18 years or over with severe CRSwNP and coexisting uncontrolled asthma receiving background mometasone furoate nasal spray. Patients were randomised 1:1 to add-on dupilumab 300 mg every 2 weeks (q2w) or omalizumab 75–600 mg q2w or q4w for 24 weeks. Due to the COVID-19 pandemic, recruitment into the EVEREST study was restricted, meaning that all intended asthma outcome measures became exploratory endpoints. Lung function measures that were assessed included changes from baseline in pre-bronchodilator FEV1 (reflecting large airway function), forced expiratory flow (25–75%; reflecting small airway function), and forced vital capacity (FVC; reflecting air trapping). Asthma control was also evaluated via ACQ-7 total scores. All endpoints were assessed over 24 weeks of treatment using mixed model for repeated measures analysis.20
In this analysis of the EVEREST trial, dupilumab provided nominally significant improvements in pre-bronchodilator FEV1 at Week 24 of treatment compared with omalizumab in patients with severe CRSwNP and coexisting uncontrolled asthma (Figure 2).20
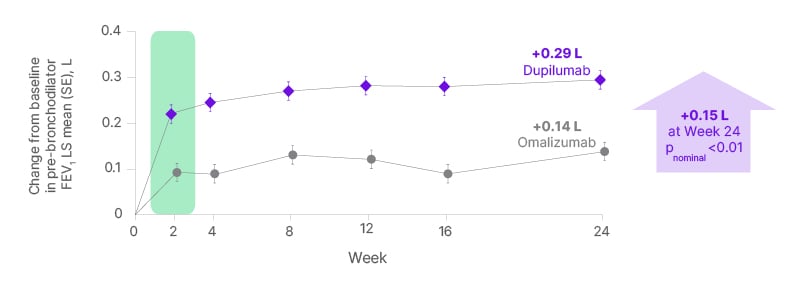
Figure 2: The EVEREST study: improvements in forced expiratory volume in 1 second with dupilumab versus omalizumab in severe chronic rhinosinusitis with nasal polyps and coexisting asthma.20
FEV1: forced expiratory volume in 1 second; LS: least squares; SE: standard error.
In addition to these increases in large airway function, similar nominally significant improvements with dupilumab versus omalizumab were also observed in other lung function measures indicative of small airway function and air trapping. Pre-bronchodilator FVC at Week 24 increased by 0.26 L in the dupilumab group versus 0.07 L in the omalizumab group, a difference of +0.19 L (pnominal=0.001). Similarly, pre-bronchodilator forced expiratory flow (25–75%) increased by 0.41 L/s and 0.21 L/s in the two treatment arms, respectively, a difference of +0.20 L/s for dupilumab over omalizumab (pnominal<0.05). Across all these lung function measures, the greater improvements in lung function and asthma control with dupilumab over omalizumab were seen as early as Week 2 and were maintained over 24 weeks of treatment.20
Dupilumab also provided nominally significant improvements in ACQ-7 total scores at Week 24 of treatment compared to omalizumab. These improvements in asthma control with dupilumab occurred as early as Week 4 and were maintained throughout the study. Overall, ACQ-7 score decreased by an additional –0.48 in the dupilumab versus omalizumab treatment groups at Week 24 (pnominal<0.001), with LS mean changes of –1.92 and –1.44, respectively.20
The Phase IV EVEREST trial marks a rare example of a head-to-head biologic study carried out in the asthma/CRSwNP space. This analysis presented at CHEST 2025 showed that dupilumab produced greater improvements in lung function and asthma control compared to omalizumab in patients with CRSwNP and uncontrolled asthma. Notably, the nominally significant improvements in lung function with dupilumab occurred in key parameters indicative of both large and small airway function and air trapping.20
COPD Exacerbations Have Less Impact on Lung Function During Dupilumab Treatment: Pooled Analysis from BOREAS and NOTUS
Dupilumab is approved in the COPD setting as add-on maintenance treatment for adults with uncontrolled COPD characterised by raised blood eosinophils on a combination of an inhaled corticosteroid (ICS), a long-acting β-2-agonist (LABA), and a long-acting muscarinic antagonist (LAMA), or on a combination of a LABA and a LAMA if ICS is not appropriate.8
This approval for COPD is based on the BOREAS and NOTUS Phase III, double-blind RCTs, which evaluated the efficacy and safety of dupilumab in adults with moderate-to-severe COPD.21-23 Across these two pivotal studies, dupilumab was shown to reduce moderate or severe exacerbations and improve lung function and QoL in patients with COPD and Type 2 inflammation.21-23
Patients with COPD enrolled into the BOREAS and NOTUS trials were between 40–85 years old, with moderate-to-severe airflow limitation and Type 2 inflammation, defined as screening blood eosinophil levels ≥300 cells/μL. These patients were already receiving treatment with ICS, LABA, and/or LAMA, and received either add-on dupilumab 300 mg or placebo every 2 weeks for 52 weeks.21-23
The post-hoc analysis, presented by Hanania at CHEST 2025, used pooled data from the BOREAS and NOTUS studies, and was designed to assess whether treatment with dupilumab reduced the impact of disease exacerbations on patients’ lung function in addition to exacerbation reduction and lung function improvement.24 COPD exacerbations are known to be associated with a significant decline in lung function, with low recovery despite resolution of the exacerbation.25,26
In total, 732 patients experienced one or more moderate or severe COPD exacerbations during the BOREAS and NOTUS study treatment periods: 338 in the dupilumab group and 394 on placebo. In order to be included in this post-hoc analysis, patients were required to have data available in the pre- (Days –59–-25), peri- (Days –24–24), and post-exacerbation (Days 25–59) periods. Changes from reference visit (Days –59–-39) in absolute and percentage predicted post-bronchodilator FEV1 and FVC were assessed as the main outcomes.24
Results of this post-hoc analysis showed that post-bronchodilator FEV1 and ppFEV1 were numerically less impaired in the pre- and peri-exacerbation periods, and more enhanced in the post-exacerbation period, with dupilumab as compared to placebo (Figure 3). Similarly, post-bronchodilator FVC and percentage predicted FVC were also numerically less impaired in the pre- and peri-exacerbation periods, and more enhanced in the post-exacerbation period, with dupilumab versus placebo. The numerical trend towards reduced lung function impact with dupilumab treatment was therefore consistent across all of the key lung function endpoints evaluated in this post-hoc analysis.24
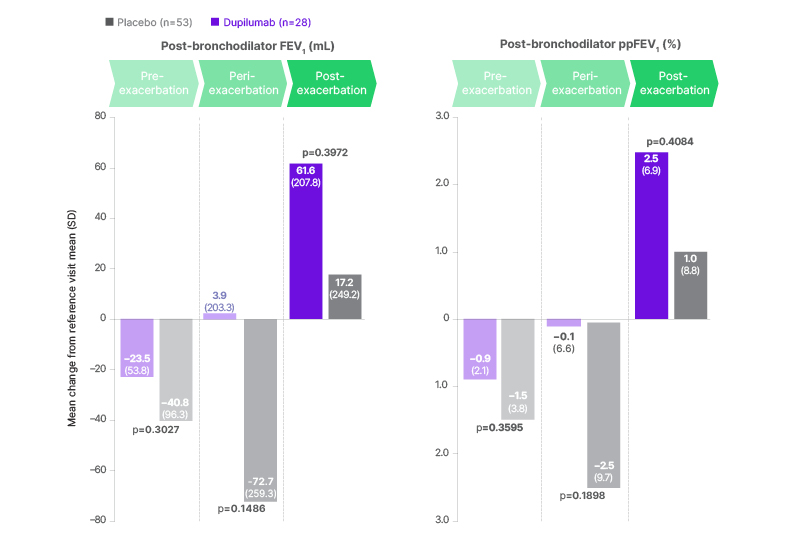
Figure 3: Post-hoc analysis: reduced impact of CODP exacerbations on lung function during dupilumab treatment versus placebo.24
FEV1: forced expiratory volume in 1 second; ppFEV1: percent predicted forced expiratory volume in 1 second.
In summary, this post-hoc analysis demonstrated that dupilumab was associated with trends towards less lung function deterioration and decline compared to placebo in patients with COPD experiencing disease exacerbations. Importantly, dupilumab was also associated with faster rebound of lung function than placebo, with treated patients showing better lung function recovery before, during, and after an exacerbation.24
Overall, these results illustrate the added value of dupilumab treatment in not only reducing the total number of COPD exacerbations and improving lung function, but also reducing the impact that these exacerbations have on patients’ lung function, thereby enabling faster recovery.24
Dupilumab Reduces the Risk of Severe Exacerbations in Patients with COPD: Results from BOREAS and NOTUS
In patients with COPD, severe exacerbations are associated with higher healthcare resource utilisation and increased morbidity and mortality risk. Preventing and effectively managing exacerbations is therefore key to improving long-term prognosis in patients with COPD.27 SCS are often prescribed for the management of COPD exacerbations; however, their prolonged or repeated use can lead to serious adverse events.28,29
The primary endpoint of the BOREAS and NOTUS studies was annualised rate of moderate or severe COPD exacerbations. In these studies, moderate exacerbations were defined as those requiring systemic steroids and/or antibiotics, while severe exacerbations were defined as those requiring hospitalisation, an ED visit >24 hours, or resulting in death.21,22 No deaths occurred in these studies.21,22 Using these outcome measures, dupilumab produced a 31% reduction in the annualised rate of moderate or severe exacerbations compared with placebo (annualised exacerbation rate: 0.794 in the dupilumab group and 1.156 in the placebo group; p<0.0001) across the two original studies.23
This post-hoc analysis of the BOREAS and NOTUS trials, presented by Bafadhel at ERS 2025, aimed to assess the efficacy of dupilumab in reducing the risk of severe exacerbations and its impact on ED visits and SCS use in patients with COPD and Type 2 inflammation.30 It employed a new endpoint for exacerbations, which is gaining increasing traction in the COPD space, namely the probability of severe exacerbation and/or ED visit, the latter defined as exacerbations requiring hospitalisation or an ED visit of any duration.31
In total in the intention-to-treat population, 60 patients on placebo and 41 on dupilumab experienced one or more severe exacerbations during the treatment period and were included in the post-hoc analysis. Baseline demographics and disease characteristics were well-balanced across these two groups.30
In this post-hoc analysis, dupilumab significantly reduced the time-to-first severe exacerbation event compared to placebo. Using the standard definition of severe exacerbation (exacerbations requiring hospitalisation, an ED visit >24 hours, or resulting in death), dupilumab achieved a 39% risk reduction at Week 52 versus placebo (hazard ratio: 0.61; p=0.0160).30
Dupilumab also delivered significant benefits versus placebo in patients with COPD using the new exacerbation endpoint of severe exacerbation event and/or ED visit. Notably, dupilumab significantly reduced the time-to-first severe exacerbation event and/or ED visit, with a 45% risk reduction at Week 52 (hazard ratio: 0.55; p=0.0010).30
In addition, dupilumab achieved a significant 38% reduction in severe exacerbation rates and/or ED visits versus placebo. The adjusted annualised rate of severe exacerbations and/or ED visits was 0.16 with placebo compared to 0.10 in those receiving dupilumab treatment (p=0.0121). Finally, patients receiving dupilumab who experienced one or more severe exacerbation events also required SCS use for significantly fewer days when compared to placebo. The adjusted annualised total SCS use was 34 days on placebo versus 20 days on dupilumab. Treatment with dupilumab therefore reduced total SCS use by 14 days compared to placebo, a reduction of 42% (p=0.0126).30
Overall, this post-hoc analysis provides more granular data supporting dupilumab’s favourable impact on the risk of exacerbations in COPD using different literature definitions of severe exacerbations. Using this new endpoint, dupilumab treatment was associated with a reduced risk of severe exacerbation events and delayed time-to-first severe exacerbation, with a reduction in ED visits and a reduced need for SCS. Collectively, these results suggest a potential benefit of dupilumab treatment in lowering disease burden and healthcare resource utilisation in patients with COPD.30
Conclusion
In summary, these new data presented at leading respiratory congresses during 2025 provide further evidence supporting the efficacy of the dual IL-4 and IL-13 inhibitor, dupilumab, in treating asthma and COPD with Type 2 inflammation. In asthma, dupilumab demonstrated efficacy in patients with coexisting ABPA, improved outcomes (including remission) in patients with severe asthma in the real-world setting, and proved superior to omalizumab in a head-to-head study in asthma with CRSwNP. In post-hoc analyses of pivotal trials in the COPD space, dupilumab reduced the risk of severe exacerbations and ED visits, and ameliorated the detrimental impact of exacerbations on patients’ lung function.
| Adverse events should be reported. Reporting forms and information for the United Kingdom can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Sanofi S.A. via the online reporting system at https://ae.reporting.sanofi/pvi-web/login or on 1800 633 1610. |







