BACKGROUND AND AIMS
Safety data on the use of biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARD) in patients with rheumatoid arthritis (RA) are reassuring. However, results from the ORAL Surveillance clinical trial have raised some concerns about a potential increase in cancer risk with the use of JAK inhibitors (JAKi) compared to TNF inhibitors (TNFi). Limited real-world data have not confirmed a higher increase in cancer risk, except for non-melanoma skin cancer,1 but additional evidence comparing all available treatment options with longer exposure is needed. For rare events with long latency periods, such as cancer, longitudinal real-world data are important, but currently limited.
In this study, the authors aim to evaluate cancer risk in patients with RA receiving bDMARDs and tsDMARDs in a large and well-established multicentre registry of targeted therapies: BIOBADASER III.2
METHODS
BIOBADASER III is a prospective cohort study based on a multicentre national registry of targeted therapies, and includes patients with RA treated with bDMARDs or tsDMARDs from 2000–2023. Patients with prior cancer were excluded from the analysis. Incidence rates were analysed, and adjusted and stratified Cox regression was used to estimate hazard ratios (HR) for all cancers excluding non-melanoma skin cancer (NMSC) and as well as for NMSC. Treatment with TNFis was used as the reference for comparison.
RESULTS
Out of a total of 4,635 patients with RA (mean age at disease onset: 55.5 years; 79% female; median follow-up: 3.6 years), 187 incident cancers were detected. The standardised incidence rate of cancer excluding NMSC per 1,000 patient-years was 7.8 for TNFis, 12 for IL6 inhibitors (IL6i), 9.2 for cluster of differentiation 20 (CD20) inhibitors (CD20i), 10.2 for JAKis, and 14.7 for cytotoxic T-lymphocyte associated protein 4 analogue (CTLA4-A). The adjusted overall HR (95% CI) for cancer excluding NMSC compared to TNFi was 1.1 (0.8–1.6) for IL6is, 0.8 (0.5–1.4) for CD20is, 1.2 (0.8–1.8) for JAKis, and 1.1 (0.8–1.6) for CTLA4-A. The adjusted overall HR (95% CI) for NMSC compared to TNFi was 0.5 (0.2–1.5) for IL6is, 0.6 (0.2–1.8) for CD20is, 0.6 (0.2–2) for JAKis, and 1.1 (0.5–2.6) for CTLA4-A. No excess cancer risk was observed in the adjusted HR based on cardiovascular risk and line of treatment for the group of all cancers excluding NMSC, or for NMSC (Figure 1).
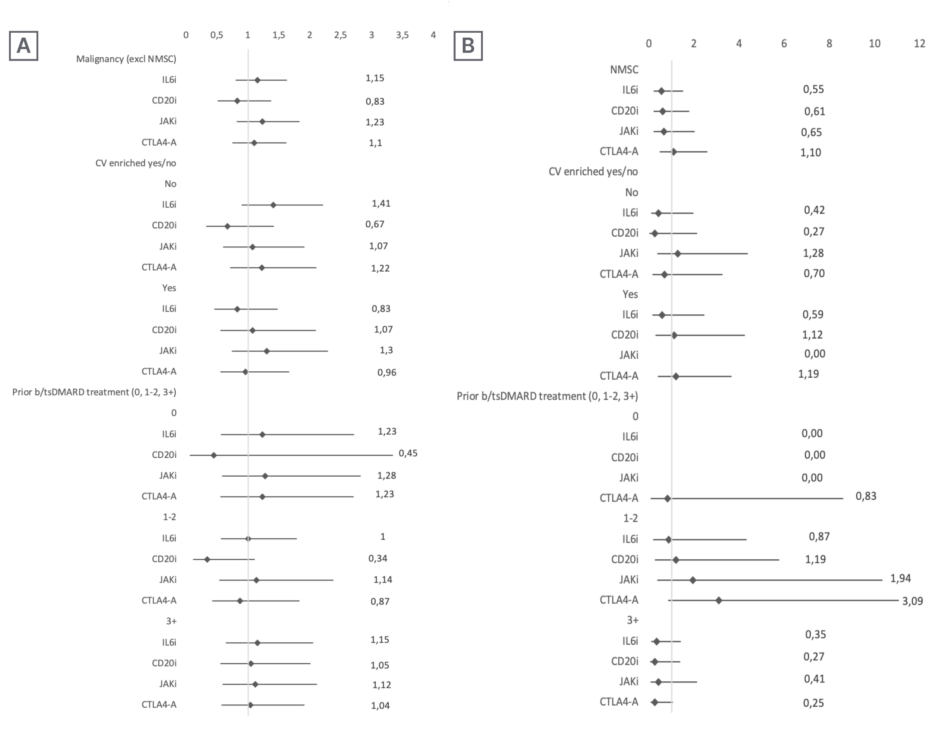
Figure 1: Cancer risk excluding non-melanoma skin cancer, and cancer risk for non-melanoma skin cancer only.
Cancer risk excluding NMSC (A), and NMSC risk (B) (overall, stratified by cardiovascular risk and by line of treatment) in patients with RA receiving targeted therapies included in BIOBADASER (hazard ratios with 95% CIs).
b/tsDMARD: biologic and targeted synthetic disease-modifying antirheumatic drugs; CD20i: cluster of differentiation 20 inhibitors; CTLA4-A: cytotoxic T-lymphocyte associated protein 4 analogue; CV: cardiovascular; Excl: excluding; HR: hazard ratio; IL6i: IL6 inhibitors; JAKi: JAK inhibitors; NMSC: non-melanoma skin cancer.
CONCLUSION
In conclusion, the authors’ analysis of the BIOBADASER registry reveals that the data have not demonstrated an increased overall cancer risk with bDMARDs or tsDMARDs compared to TNFis, reinforcing that the cancer safety profile of these therapies is generally reassuring. Nonetheless, ongoing vigilance through long-term observational studies and further research is essential to monitor potential risks associated with specific therapies and cancer subtypes.